
Concept explainers
(a)
Interpretation: For the given
Concept introduction:
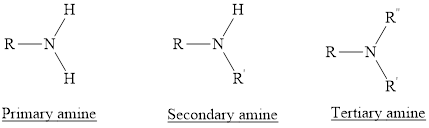
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.
(a)
Answer to Problem 47PS
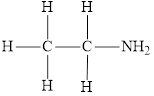
The molecular formula of the given amine is
Explanation of Solution
The molecular formula of the given amine can be written by using its given name ethylamine.
The ethyl group
Therefore molecular formula will have on ethyl group and two hydrogen atoms attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,

(b)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(b)
Answer to Problem 47PS
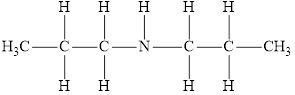
The molecular formula of the given amine is
Explanation of Solution
The molecular formula of the given amine can be written by using its given name dipropylamine.
The two propyl groups
It is secondary amine as two alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have two propyl groups and one hydrogen atom attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,

(c)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(c)
Answer to Problem 47PS
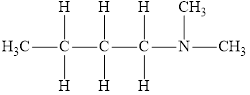
The molecular formula of the given amine is
Explanation of Solution
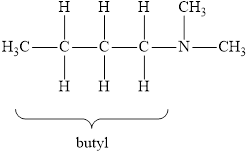
The molecular formula of the given amine can be written by using its given name butyl dimethylamine.
The two methyl groups
It is tertiary amine as three alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have two methyl groups, one butyl group and no hydrogen atom attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,
(d)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(d)
Answer to Problem 47PS
The molecular formula of the given amine is
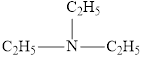
Explanation of Solution
The molecular formula of the given amine can be written by using its given name triethylamine.
The three ethyl groups
It is tertiary amine as three alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have three ethyl groups, no hydrogen atom attached to nitrogen atom, that is
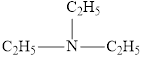
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity
- (i) Draw the structure of any amine and give the IUPAC name of that amine. (i) Classify the amine in your answer provided in (i) above (iii) Draw the structure of ethyl butanoate and name the functional group. (iv) Give the IUPAC name of the following compound and name the functional group:arrow_forwardTRUE OR FALSE (a) There are three amines with the molecular formula C3H9N. (b) Aldehydes, ketones, carboxylic acids, and esters all contain a carbonyl group. (c) A compound with the molecular formula of C3H6O may be either an aldehyde, a ketone, or a carboxylic acid. (d) Bond angles about the carbonyl carbon of an aldehyde, a ketone, a carboxylic acid, and an ester are all approximately 109.5°. (e) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (f) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forward(a) Compound Z is a tertiary aromatic amine with the formula, C8H11N. Provide a chemical structure for compound Z. (b)nDraw the structure of the product formed exclusively when nitrous acid reacts with Z.arrow_forward
- Draw a structural formula for each amine. (a) 2-Butanamine (b) 1-Octanamine (c)2,2-Dimethyl-1-propanaminearrow_forward(a) Draw the structures for the eight constitutional isomers of molecular formula C 4H 11N. (b) Give the systematic name for each amine. (c) Identify the chirality center present in one of the amines.arrow_forwardDraw the condensed formula or skeletal structural formula for the following compounds: (a) 2,3-dimethylpentanoic acid (b) Ethyl pentanoate (c) N,N-dimethyl-1-propanaminearrow_forward
- Describe the water solubility of amines in relation to theircarbon chain length.arrow_forwardWhich of the following is not an oxygen-containing organic compound? (A) amine (B) acid halide (c) ketone D etherarrow_forward(a) Write the molecular formula of acetaminophen (see Fig. 7.34b).(b) A tablet of Extra Strength Tylenol contains 500 mg acetaminophen. Calculate the chemical amount (in moles) of that compound in the tablet.arrow_forward
- Draw and name compounds that meet these descriptions:(a) Three different amides with the formula C5H11NO(b) Three different esters with the formula C6H12O2arrow_forwardIn each pair, indicate the compound that is more soluble in water. (a) trimethylamine or propylamine (b) tripropylamine or ethylpropylamine (c) butylamine or ethylamine (d) propylamine or butanearrow_forwardWrite a condensed structural formula for each of the following:(a) an acid with the formula C4H8O2, (b) a cyclicketone with the formula C5H8O, (c) a dihydroxy compoundwith the formula C3H8O2, (d) a cyclic ester with theformula C5H8O2.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





