
a)

Interpretation:
Electrostatic potential maps of formaldehyde is given.The nature of the formldehyde carbon atom whether electrophilic or nucleophilic is to be stated and explained.
Concept introduction:
Electrostatic potential maps are used to identify at a glance the electron rich or electron poor atoms in molecule. In them the electron rich regions are shown in red and electron poor region are shown in blue.
To state and explain:
From the electrostatic potential map of formaldehyde the nature of the formldehyde carbon atom, whether it is electrophilic or nucleophilic.
b)
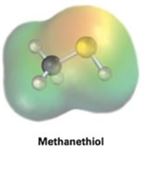
Interpretation:
Electrostatic potential map of methanethiol (CH3SH) is given. The nature of the sulfur atom in methanethiol, whether electrophilic or nucleophilic, is to be stated and explained.
Concept introduction:
Electrostatic potential maps are used to identify at a glance the electron rich or electron poor atoms in molecule. In them the electron rich regions are shown in red and electron poor region are shown in blue.
To state and explain:
From the electrostatic potential map of methanethiol, whether the sulfur atom in it is electrophilic or nucleophilic.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Industrial chemistry: Provide a mechanism for the hydrocracking of n-Heptane in the presence of hydrogen gas to isopentane and ethane.arrow_forwardA certain hydrocarbon had the molecular formula C18H30 and contained two triple bonds. Ozonolysis gave CH₂(CH₂)CO₂H and HO₂CCH₂CH₂CO₂H as the only products. Draw a reasonable structure for this hydrocarbon. Click and drag to start drawing a structure.arrow_forwardCore 2 (a) The structure of limonene is shown below. CH₂ CH₂ CH₂ CH CH₂ (i) What is the molecular formula of limonene? **********‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒ (II) Some limonene was added to a few drops of aqueous bromine. What colour change would you see in the aqueous bromine? ************ (iii) What feature of a limonene molecule is responsible for this colour change? (iv) Name the two substances formed when limonene is burnt in an excess of oxygen.arrow_forward
- Nitration of an aromatic ring involves an electrophilic substitution reaction. Draw the structure of the electrophile that is attacked by the aromatic ring and the intermediate formed after attachment of the electrophile to the ring. Be sure to show formal charges.arrow_forwarda) If the pH value of an aqueous solution of trimethylamine [(CH3) 3N] is 10.75, what should be the molarity of this solution? (CH3) 3N + H2O ↔ (CH3) 3NH + + OH-, Kb = 6.3 × 10-5 b) What will be the pH of the solution prepared by dissolving 8.35 g of aniline hydrochloride (C6H5NH3 + Cl-) in 750 mL of 0.215 M aniline (C6H5NH2)? Is this solution an effective buffer? Explain (Kb = 7.4 × 10-10 for aniline, C: 12.0 g / mol, H: 1.0 g / mol, N: 14.0 g / mol, Cl: 35.4 g / mol) .arrow_forwardIntramolecular H-bonding (IHB) are interactions that occur within one single molecule. IHB is also known as chelation, since it involves ring formation. Which of the following is (are) stabilized by IHB? * QH Çi OH -NO2 (a) Cl-Ç–Ç–H (b) či ÓH OH (с) CH, —С—СH—С—ОС,Н, CH,OH (d) | CH,OH D B Aarrow_forward
- a) If the pH value of an aqueous solution of trimethylamine [(CH3)3N] is 10.75, what should be the molarity of this solution? (CH3)3N + H2O ↔ (CH3)3NH+ + OH-, Kb = 6,3 × 10-5 b) What will be the pH of the solution prepared by dissolving 8.35 g of aniline hydrochloride (C6H5NH3+Cl-) in 750 mL of 0.215 M aniline (C6H5NH2)? Is this solution an effective buffer? Explain (Kb = 7,4 × 10-10 for aniline, C: 12.0 g / mol, H: 1.0 g / mol, N: 14.0 g / mol, Cl: 35.4 g / mol) .arrow_forward2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forwardRadicals and carbocations are electrophiles. Define how and why ?arrow_forward
- Chlorination of 2-butanone yields two isomeric products, each having the molecular formula C4H7ClO. (a) What are these two compounds? (b) Write structural formulas for the enol intermediates that lead to each of these compounds. (c) Using curved arrows, show the flow of electrons in the reaction of each of the enols with Cl2.arrow_forwardIn each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forwardThe reaction of ozone with 2-butene leads to -10 formation of aldehyde + ketone 0 aldehyde + alcohol 0 two molecules of carboxylic acid 0arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
