
Concept explainers
(a)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid
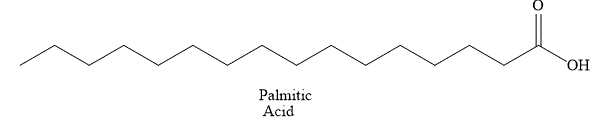
Figure 1
The general name of the above fatty acid is palmitic acid. Palmitic acid has a long aliphatic chain of
The fatty acid shown in Figure 1 is a saturated fatty acid and it is solid at room temperature.
(b)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 2
The general name of the above fatty acid is lenoleic acid. The structural formula of lenoleic acid shows that there are two double bonds in the carbon chain. Therefore, lenoleic acid is an unsaturated fatty acid. The side chain in unsaturated fatty acids possess less van der Waal’s interactions between side chains of adjacent molecule. Therefore, the unsaturated fatty acid is a liquid at room temperature.
The fatty acid shown in Figure 2 is an unsaturated fatty acid and it is a liquid at room temperature.
(c)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 3
The general name of the above fatty acid is palmitolic acid. The structural formula of palmitolic acid contains double bonds within the fatty acid chain. Therefore, palmitolic acid is an unsaturated fatty acid. The side chain in unsaturated fatty acids possess less van der Waal’s interactions between side chains of adjacent molecule. Therefore, the unsaturated fatty acid is a liquid at room temperature.
The fatty acid shown in Figure 3 is an unsaturated fatty acid and it is a liquid at room temperature.
(d)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 4
The general name of the above fatty acid is lauric acid. Lauric acid has a long aliphatic chain of
The fatty acid shown in Figure 4 is a saturated fatty acid and it is a solid at room temperature.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- 1. What are carboxylic acids? What is their type formula? 9. Why are they called fatty acids? 10. How may acids be obtained from alcohols and aldehydes?arrow_forwardIs CH3F or CH3CH2OH more soluable in water.arrow_forwardof the product. Name the reactant and the product. a) Cy-CH-C-OH b) CHy-CH-CH-CHy c) 4. Complete the following intermolecular dehydration reactions for alcohols. Draw the structure of the product. Name the reactant and the product. a) CH3-CHS-OH b) CH-CH-CH_-OH 5. Complete the following oxidation reactions for alcohols. Draw the structure of the product. Name the reactant and identify the type of compound formed in the product. a) CHっ-CHュ-CH-Cs OH b) c) Co] Ho--cgCらarrow_forward
- . The following chemical reactants produce the ester ethylethanoate (C4H8O2):C2H6O + CH3COOHWhat type of reaction occurs to make ethyl ethanoate?a. condensationb. hydrolysisc. combustiond. acid-base reactionarrow_forwardb) Ho わ-fャコーfつ Koc 5. Complete the following oxidation reactions for alcohols. Draw the structure of the product. Name the reactant and identify the type of compound formed in the product. CHっーCHューC OH b) CHy CH3arrow_forwardWhich would be the most important type of intermolecular force between fat molecules and petroleum ether? Explain your reasoning. Also note that petroleum ether is a mix of hydrocarbons (C6-16H12-34).arrow_forward
- 23. Draw the structure of the ester formed between the following fatty acid and the alcohol. O CH3 (CH2)16- COH and CH3(CH2)12 CH₂OH What is the name of this lipid?arrow_forwardExplain why diethyl ether's boiling point is lower than the boiling point of butan-1-ol. Support your answer using the concept of intermolecular forces of attraction. Using the MEP model of the molecules, illustrate the formation of intermolecular forces of attraction that is present in each chemical system.arrow_forward3. Identify and label the parts of this soap molecule. Explain how molecules of soap remove oil and grease by the formation of micelles. H2 H2 H2 CH3 O`Na*arrow_forward
- LSaxw_byUjt4NKKTEABPFImknTAVIxeSATH3-MO9AEbrFOXuga ponse TUIse The structure of glycogen is very similar to that of amylose Secondary alcohols are readily oxidized with common oxidizing agents to carboxylic acids Amines react with strong acids such as HCI, to form ammonium salts In general, oils come from animal sources and fats from vegetable sources Amines are weak bases, they are considerably more basic than alcohols, and water Carboxylic acids are less acidic than alcohols Steroids are lipids that do not contain fatty acids Alkylamines have boiling points are higher than those of alkanes, but lower than those of alcohols 4- Tio druck & 1 8. 9. Y ! OX { Hi J K : NI pause O O O O O O O O OOarrow_forwardWhat are fats and oils? a. ketones formed of two or more esters b. any large molecular weight organic compound that has been saturated c. esters formed from glycerol and three carboxylic acids d. polymers of repeating aromatic hydrocarbonsarrow_forward● Arrange these compounds in order of increasing boiling point. Heptane HO. LOH 1,5-Pentanediol Propanol 1-Hexanol LOHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





