
Chemistry In Context
9th Edition
ISBN: 9781259638145
Author: Fahlman, Bradley D., Purvis-roberts, Kathleen, Kirk, John S., Bentley, Anne K., Daubenmire, Patrick L., ELLIS, Jamie P., Mury, Michael T., American Chemical Society
Publisher: Mcgraw-hill Education,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 46Q
Here are two scanning electron micrograph images of particulate matter, courtesy of the National Science Foundation and researchers at Arizona State University. The first is of a soil particle and the second of a rubber particle, and each is about 10 μm in diameter.
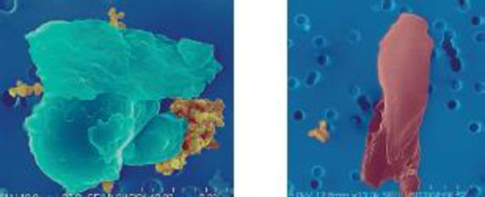
(both) Source: National Science Foundation, Courtesy of Xin Hua & James Anderson
- a. Suggest a likely source of the rubber particle. Name two other substances that might contribute PM to the air.
- b. The soil particle is composed mainly of silicon and oxygen. What other elements are commonly present in the rocks and minerals in Earth’s crust?
- c. What about these photographs suggests that these particles would inflame your blood vessels?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Suppose you have an 8-hour ozone value of 0.088 ppm, and a PM2.5 value of 35.7 ug/m3. The AQI value is Blank 1 and the
responsible pollutant is Blank 2. According to the AQI scale, this falls under the category Blank 3.
Note: for the pollutant, choose between ozone and PM2.5
These Breakpoints.
.equal
this AQI
...and this
...
category
Air Quality Index
Levels of Health
Concern
Numerical
Value
PM30
PM25
(48/m³)| (48/m²')
SO2
(ppb)
1-hour
NO2
(ppb)
1-hour
CO
AQI
(ppm)
(ppm)
1-hour
(ppm)
8-hour
8-hour
24-hour
24-hour
Good
0 to 50
0.000 -
0.0 – 12.0 0- 54
0.0 - 4.4
0- 35
0- 53
0- 50
Good
0.054
Moderate
51 to 100
55 - 154
4.5 - 9.4
54 - 100
51 - 100
0.055 -
0.070
12.1 -
36 - 75
Moderate
35.4
Unhealthy for
Sensitive Groups
101 to 150
155 - 254 9.5 - 12.4 76 - 185|101 - 360 101 - 150
Unhealthy for
Sensitive Groups
0.071 -
0.125-
35.5 -
0.085
0.164
55.4
255 - 354
Unhealthy
151 to 200
(186 -
304)*
0.086 -
0.165 -
(55.5 -
12.5 -
361 - 649 151 - 200
Unhealthy
0.105
0.204
150.4)
15.4
Very Unhealthy…
Suppose you have an 8-hour ozone value of 0.088 ppm, and a PM2.5 value of 35.7 pg/m3. The AQI value is Blank 1 and the
responsible pollutant is Blank 2. According to the AQI scale, this falls under the category Blank 3.
Note: for the pollutant, choose between ozone and PM2.5
These Breakpoints.
.equal
this AQI
..and this
...
category
Air Quality Index
Levels of Health
Numerical
Value
PM25
PM30
(H8/m') (Hg/m)
Co
SO2
NO2
AQI
Concern
(ppm)
1-hour
(ppm)
(ppm)
(ppb)
(ppb)
1-hour
8-hour
24-hour 24-hour
8-hour
1-hour
Good
O to 50
0.000 -
0.0-12.0
0- 54
0.0 - 4.4
0- 35
0 - 53
0- 50
Good
0.054
Moderate
51 to 100
0.055 -
12.1-
55 - 154 4.5 -9.4
36 - 75
54 - 100
51 - 100
Moderate
0.070
35.4
Unhealthy for
Sensitive Groups
101 to 150
0.125 -
155 - 254 9.5 - 12.4 76 - 185101 - 360 101 - 150
Unhealthy for
Sensitive Groups
0.071 -
35.5 -
0.085
0.164
55.4
0.086 -
0.165 -
(55.5 -
255 - 354
12.5 -
(186 -
Unhealthy
151 to 200
361 - 649 151 - 200
Unhealthy
0.105
0.204
150.4)
304)*
15.4
Very Unhealthy
201 to…
58. Apply Graph mass versus volume for the data given in the table.What is the
N, 20.95% O
0234 Ar and 0.04% CO, and other gases.
51. infer from Figure 17 how long the ozone hole lasts.
58. Apply Graph mass versus volume for the data given in the table wi
slope of the line?
7.5
12
15
22
Volume (cm³)
Mass (g)
24.1
38.5
48.0
70.1
Chapter 2 Solutions
Chemistry In Context
Ch. 2.1 - Prob. 2.2YTCh. 2.2 - Prob. 2.3YTCh. 2.2 - The air is different in a pine forest, a bakery,...Ch. 2.3 - Scientific Practices More Oxygen ? We live in an...Ch. 2.4 - Prob. 2.6YTCh. 2.4 - Prob. 2.7YTCh. 2.7 - Prob. 2.8YTCh. 2.7 - Skill Building Mother Eats Peanut Butter Many...Ch. 2.8 - Prob. 2.10YTCh. 2.9 - Prob. 2.11YT
Ch. 2.9 - Sulfur dioxide (SO2) is released in the air when...Ch. 2.9 - Prob. 2.13YTCh. 2.10 - Prob. 2.14YTCh. 2.10 - Prob. 2.15YTCh. 2.11 - Prob. 2.16YTCh. 2.12 - Prob. 2.17YTCh. 2.12 - Prob. 2.18YTCh. 2.13 - Prob. 2.19YTCh. 2.13 - Prob. 2.20YTCh. 2.13 - Prob. 2.21YTCh. 2.13 - Prob. 2.22YTCh. 2.14 - Prob. 2.24YTCh. 2.14 - Summarize what you have learned about ozone...Ch. 2.15 - Prob. 2.27YTCh. 2.15 - Prob. 2.28YTCh. 2 - Scientific Practices Footprints in the Air Hiking...Ch. 2 - Prob. 1QCh. 2 - Prob. 2QCh. 2 - Identify three sources of particulate matter found...Ch. 2 - Prob. 4QCh. 2 - Gases found in the atmosphere in small amounts...Ch. 2 - Hydrocarbons are important fuels that we burn...Ch. 2 - Prob. 7QCh. 2 - If you had a sample of 500 particles of air, how...Ch. 2 - Count the atoms on both sides of the equation to...Ch. 2 - Prob. 10QCh. 2 - Prob. 11QCh. 2 - These questions relate to the combustion of...Ch. 2 - Balance the following equations in which ethane...Ch. 2 - Prob. 14QCh. 2 - Prob. 15QCh. 2 - Prob. 16QCh. 2 - Prob. 17QCh. 2 - Name the following nitrogen-containing compounds:...Ch. 2 - Prob. 19QCh. 2 - A carbon monoxide detector will go off if the...Ch. 2 - Prob. 21QCh. 2 - Nail polish remover containing acetone was spilled...Ch. 2 - Prob. 23QCh. 2 - Prob. 24QCh. 2 - Prob. 25QCh. 2 - Prob. 26QCh. 2 - A headline from the Anchorage Daily News in Alaska...Ch. 2 - Consider how life on Earth would change if the...Ch. 2 - Prob. 29QCh. 2 - Undiluted cigarette smoke may contain 23% CO. a....Ch. 2 - Prob. 31QCh. 2 - Prob. 32QCh. 2 - Prob. 33QCh. 2 - Here are air quality data for the last week of...Ch. 2 - Prob. 35QCh. 2 - Prob. 36QCh. 2 - Prob. 37QCh. 2 - Prob. 38QCh. 2 - Prob. 39QCh. 2 - Consumers now can purchase paints that emit only...Ch. 2 - Prob. 41QCh. 2 - Prob. 42QCh. 2 - Prob. 43QCh. 2 - Mercury, another serious air pollutant, is not...Ch. 2 - The EPA oversees the Presidential Green Chemistry...Ch. 2 - Here are two scanning electron micrograph images...Ch. 2 - Prob. 47QCh. 2 - Prob. 48QCh. 2 - You may have admired the beauty of hardwood...Ch. 2 - Prob. 50Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Give one example from main group chemistry that illustrates each of the following descriptions: (a) Covalent ne...
General Chemistry: Atoms First
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Marie Curie was born in Poland but studied and carried out her research in Paris. In 1903, she shared the Nobel Prize in Physics with H. Becquerel and her husband Pierre for their discovery of radioactivity. (In 1911 she received the Nobel Prize in Chemistry for the discovery of two new chemical elements, radium and polonium, the latter named for her homeland, Poland.) They and others observed that a radioactive substance could emit three types of radiation: alpha (), beta (), and gamma (). If the radiation from a radioactive source is passed between electrically charged plates, some particles are attached to the positive plate, some to the negative plate, and others feel no attraction. Which particles are positively charged, which are negatively charged, and which have no charge? Of the two charged particles, which has the most mass? Radioactivity. Alpha (), beta I(), and gamma () rays from a radioactive element are separated by passing them between electrically charged plates.arrow_forwardChapter #3, Question #13: Consider the following data for total atmospheric column ozone measurements (as DU) at three locations around the Earth, obtained using the TOMS in 2001. January 15 april 15 july 15 october 15 Tierra del fuego (Chilie, argentina) 323 261 339 206 Nairobi (Kenya) 234 273 266 (Aug. 15) 266 Kiev (Ukraine) 321 420 314 273 Assume that these are typical of values that might be obtained in any other year, and discuss the trends as you move down the columns and along the rows, in terms of your knowledge of stratospheric ozone behavior.arrow_forwardMind Tap - Cengage Learning X om/ilrn/takeAssignment/takeCovalent Activity.do?locator=assignment-take me teaching and le X [Review Topics] [References] Use the References to access important values if needed for this question. An 1.11 mol sample of nitrogen gas at a temperature of 10.0°C is found to occupy a volume of 27.5 liters. The pressure of this gas sample is atm. Submit Answer 888 F4 + A FS Retry Entire Group 8 more group attempts remaining Cengage Learning Cengage Technical Support MacBook Air VA DII DD Previousarrow_forward
- The ozone concentration at a monitoring site is measured as 0,11 ppm, at 25 °C and 1 atm. What is the concentration (in pg/m3) at 25 °C and 1 atm? (MW of ozone is 48)a. 155 pg/m3b. 190 pg/m3c. 215 pg/m3d. 260 pg/m3arrow_forwardYou are debating with a politician who claims that global warming is a hoax. Which of the following statements could you use to counter their arguments? Select one or more: O a. We are increasing the amount of CO2 a greenhouse gas, in our atmosphere O b. Developing countries are consuming the most energy by capita. O c. Cows emit CFCS, which is a greenhouse gas and contributes to global warming. O d. The change in weather is due to ozone destruction O e. Global warming is causing polar ice caps to melt, causing a rise in sea levels that can wipe out coastal cities. O f. We can reduce the global temperature rise of 2 degree Celsius if industrial countries reduce their carbon emission by 75% by 2050. Please select all the actions that lead to the 2014 Flint water public health crisis and continues to be a problem today. Select one or more: O a. Addition of ferric chloride to the water sourced from the Flint River made the water more corrosive. O b. Orthophospates were not originally used…arrow_forward2 What is reservior in environmental chemistry? What are O1D (O singlet D) and O3P( O priplet P)? Not sure if it's written in this way.arrow_forward
- 2.) It’s a bad day in the lab! Two students are doing experiments. Each is 20 feet away from the professor. At the same time, each of them lets the same amount of a smelly gas into the room. One of them releases ammonia, NH3, and the other releases SO2. NH3 has a pungent odor, and SO2 smells like rotten eggs. The professor has no idea that this has happened, until she smell the first gas. Which chemical will the professor smell first? (NH3 or SO2) . If the professor starts to smell the first gas 42. seconds after the gas is released, how long will it take her to smell the second gas? sec. * Note: It is unsafe practice to work with these chemicals in an open lab.arrow_forwardScientists noticed that the ozone layer was thinning.What was occurring at the same time?arrow_forward- What chemical species in the atmosphere acts like nature’s cleaning agent? Give the symbol and name. -What is one physical mechanism (i.e., not a chemical reaction) through which trace gases can be removed from the atmosphere?arrow_forward
- 1.Methane was combusted at 100.0 degrees C and 1.00 atm. The following data table was used to collect and record data: Item Mass Before (g) Mass After (g) Volume (L) Methane 774 x x Oxygen XS x x Carbon dioxide x ? ? Water Vapor x ? ? CH4 + O2 ---> How many grams of water vapor is formed as a result of the reaction? How many liters of carbon dioxide is formed? _______g H2O _______ L CO2 Blank 1: Blank 2:arrow_forwardPlease help me fill in the highlighted. I don't know. Please explain how to get the answers. Thank you in advance.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY