
Concept explainers
(a)
Interpretation:
The resonance structures with an octet about the central atom and a resonance structure that has minimum formal charges for the following structure has to be determined.
Concept Introduction:
A covalent bond is a bond that is formed from the mutual sharing of electrons between atoms. Lewis structures are representations of the covalent bond. In this, Lewis symbols show how the valence electrons are present in the molecule.
Steps to write Lewis structures are as follows:
1. The skeleton structure with single bonds between all bonded atoms has to be written
2. Sum the valence electrons of the atoms in the molecule.
(a) For cations, one electron is subtracted for each positive charge.
(b) For anions, one electron is added for each negative charge.
3. Subtract two electrons from total number of valence electrons for each bond in the skeleton structure.
4. Count the number of electrons required to satisfy the octet rule for each atom in the structure. If the number of electrons needed is less than the number remaining, add one bond for every two electrons needed between atoms to attain an octet.
5. The remaining electrons are placed as lone pairs on atoms that need them to satisfy the octet rule.
The formula to calculate formal charge of atom is,
Some molecules do not have only one Lewis structure. The Lewis structures that differ only in the arrangement of multiple bonds are called resonance structures.
Resonance structure comprises of two or more Lewis Structures that describes the arrangement of bond of a single species and include fractional bonds and fractional charges.
(a)
Answer to Problem 9.80QE
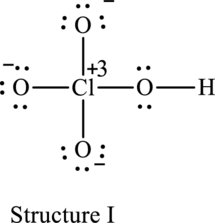
The resonance structure that has an octet around central atom is as follows:
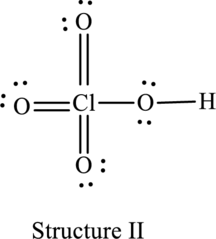
The resonance structure that minimizes formal charge is as follows:
Explanation of Solution
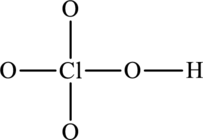
The skeleton structure is as follows:
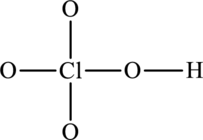
The resonance structures are as follows:
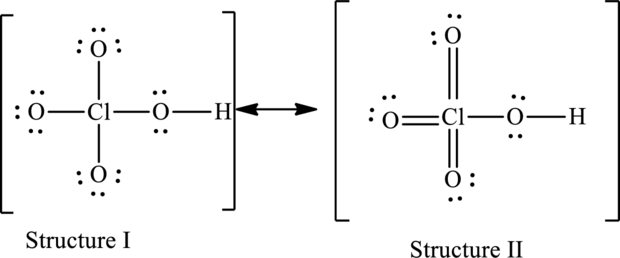
For structure I:
Substitute 7 for valence electrons, 4 for the number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on fourth oxygen atom.
For structure II:
Substitute 7 for valence electrons, 0 for the number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on fourth oxygen atom.
Possible resonance structures are as follows:
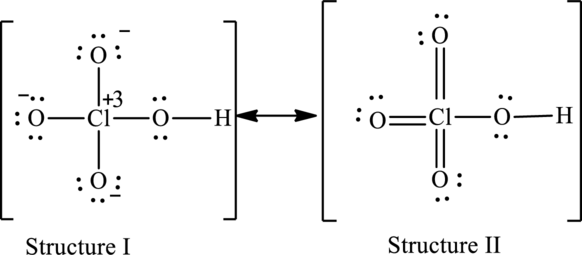
Hence, structure I has an octet around central atom and structure II minimizes the formal charge.
(b)
Interpretation:
The resonance structures with an octet about the central atom and a resonance structure that has minimum formal charges for the following structure has to be determined.
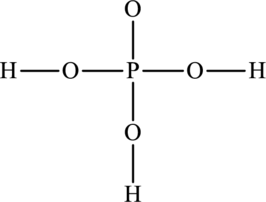
Concept Introduction:
Refer to part(a).
(b)
Answer to Problem 9.80QE
The resonance structure that has an octet around central atom is as follows:
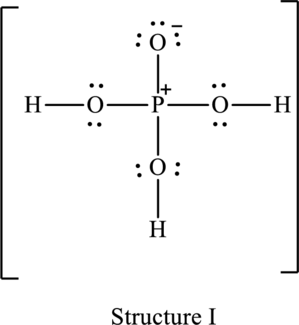
The resonance structure that minimizes formal charge is as follows:
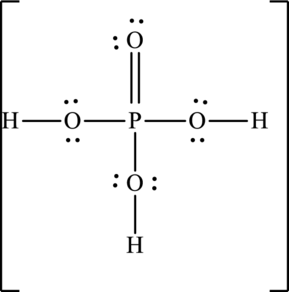
Explanation of Solution
The skeleton structure is as follows:
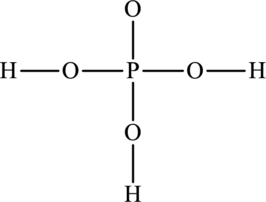
The resonance structures are as follows:
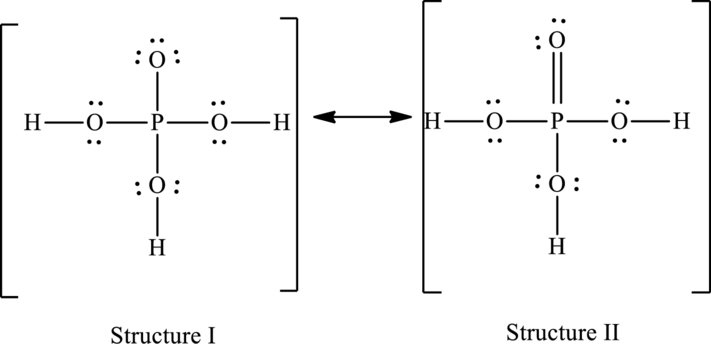
For structure I:
Substitute 5 for valence electrons, 0 for the number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on fourth oxygen atom.
For structure II:
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 10 for the number of shared electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on fourth oxygen atom.
Possible resonance structures are as follows:
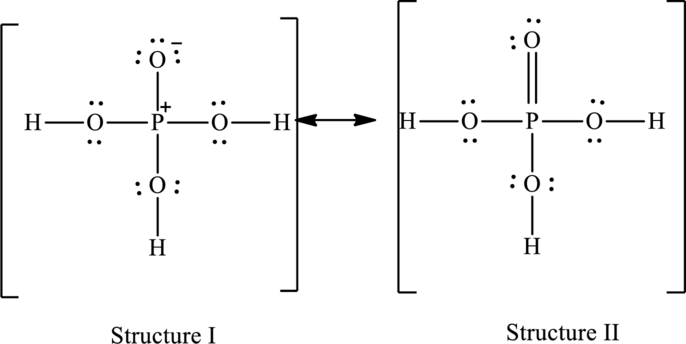
Hence, structure I has an octet around central atom and structure II minimizes the formal charge.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: Principles and Practice
- Chemistry (a) Write three more resonance structures for each of compounds 1 and 2. (b) In each of compounds 1 and 2, determine which resonance structure contributes the most and explain your answer. (c) Are the 3/4 structures resonance structures or different compounds? Same question for 5/6 structures. Explain your answers.arrow_forwardUsing just a periodic table (not a table of electronegativities), decide which of these is likely to be the most polar bond. Explain your answer! (a) C-F (b) S-F (c) Si-F (d) O-Farrow_forwardDraw Lewis electron dot diagrams for the following species, indicating formal charges and resonance diagramswhere applicable.(a) H3NBF3 (b) CH3COO- (acetate ion) (c) HCO3- (hydrogen carbonate ion)arrow_forward
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gasarrow_forwardA stable triatomic molecule can be formed that containsone atom each of nitrogen, sulfur, and fluorine. Threebonding structures are possible, depending on which is thecentral atom: NSF, SNF, and SFN.(a) Write a Lewis diagram for each of these molecules,indicating the formal charge on each atom.(b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom?(c) Does consideration of the electronegativities of N, S,and F from Figure 3.18 help rationalize this observedstructure? Explain.arrow_forward(a) Draw the two resonance structures of the molecule below (ignore structures that violate the octet rule): (b) For the above question, indicate which resonance structure (either the one given, or one that you drew) would be the most significant for this compound. Briefly state the reason for your choice.arrow_forward
- (a) -x- (b) Identify the main group that the element X belongs to in each of the following Lewis structures. For the types of molecule shown. -x-öarrow_forwardKeeping the same atomic connections and moving only electrons, write a more stable Lewis structure for each of the following. Be sure to specify formal charges, if any, in the new structure. (g) (h) (i)arrow_forwardIn which of the following ions does carbon have a formal charge? Explain your answer. A) I B) II C) III D) None of thesearrow_forward
- Draw a Lewis Structure for each of the following species and assign formal charge where appropriate. Using electronegative values from the period table that was provided identify polar covalent bonds and label the atoms δ+ and δ−. For each of the molecules indicate whether or not it has a dipole moment. (a)CH5N (b) HCN (c) H2CO (d) CH3NC(e) CH3SOCH3 (f) H6BNarrow_forwardDraw a Lewis structure for each of the following molecule: (a) chlorodifluoromethane, CHCIF2 (b) propanoic acid C2H5CO2H (basic structure pictured below) (c) acetonitrile, CH3CN ( the framework is H3C-C-N) (d) allene, H2CCCH2arrow_forwardFinish the following questions. ((a) Draw all of the possible Lewis structures (including reasonance structures) of the following compounds.(b) Label the formal charge for each atom.(c) Determine which resonance structure(s) is(are) the better/best and briefly explain. ClO2F2+arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





