
Concept explainers
The compound hexaazaisowurtzitane is one of the highest-energy explosives known (C & E News, Jan. 17, 1994, p. 26). The compound, also known as CL-20, was first synthesized in 1987. The method of synthesis and detailed performance data are still classified because of CL-20’s potential military application in rocket boosters and in warheads of “smart” weapons. The structure of CL-20 is
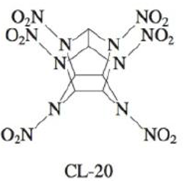
In such shorthand structures, each point where lines meet represents a carbon atom. In addition, the hydrogens attached to the carbon atoms are omitted; each of the six carbon atoms has one hydrogen atom attached. Finally, assume that the two O atoms in the NO2 groups are attached to N with one single bond and one double bond.
Three possible reactions for the explosive decomposition ofCL-20 are
i.
ii. C6H6N12O12(s) → 3CO(g) + 3CO2(g) + 6N2(g) + 3H2O(g)
iii. C6H6N12O12(s) → 6CO2(g) + 6N2(g) + 3H2 (g)
a. Use bond energies to estimate ∆E for these three reactions.
b. Which of the above reactions releases the largest amount of energy per kilogram of CL-20?
(a) (i)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is either gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 163CP
The change in energy
Explanation of Solution
Given
The chemical reaction involved is,
Formula
Energy for reactants,
The total energy
For products,
The total energy
The change in energy
(ii)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is either gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 163CP
The change in energy
Explanation of Solution
Given
The chemical reaction involved is,
Formula
Energy for reactants,
The total energy
For products,
The total energy
The change in energy
(iii)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is either gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 163CP
The change in energy
Explanation of Solution
Given
The chemical reaction involved is,
Formula
Energy for reactants,
The total energy
For products,
The total energy
The change in energy
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
(b)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is either gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The reaction that releases the larger amount of energy per kilogram of
Answer to Problem 163CP
The reaction (iii) releases the largest amount of energy per kilogram of
Explanation of Solution
One mole of
In case of the (i) reaction,
Hence,
In case of the (ii) reaction,
Hence,
In case of the (iii) reaction,
Hence,
The reaction (iii) releases the largest amount of energy per kilogram of
The third stated reaction releases the largest amount of energy per kilogram of
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
- Write all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardBond Enthalpy When atoms of the hypothetical element X are placed together, they rapidly undergo reaction to form the X2 molecule: X(g)+X(g)X2(g) a Would you predict that this reaction is exothermic or endothermic? Explain. b Is the bond enthalpy of X2 a positive or a negative quantity? Why? c Suppose H for the reaction is 500 kJ/mol. Estimate the bond enthalpy of the X2 molecule. d Another hypothetical molecular compound, Y2(g), has a bond enthalpy of 750 kJ/mol, and the molecular compound XY(g) has a bond enthalpy of 1500 kJ/mol. Using bond enthalpy information, calculate H for the following reaction. X2(g)+Y2(g)2XY(g) e Given the following information, as well as the information previously presented, predict whether or not the hypothetical ionic compound AX is likely to form. In this compound, A forms the A+ cation, and X forms the X anion. Be sure to justify your answer. Reaction: A(g)+12X2(g)AX(s)The first ionization energy of A(g) is 400 kJ/mol. The electron affinity of X(g) is 525 kJ/mol. The lattice energy of AX(s) is 100 kJ/mol. f If you predicted that no ionic compound would form from the reaction in Part e, what minimum amount of AX(s) lattice energy might lead to compound formation?arrow_forwardThe equation for the combustion of gaseous methanol is 2 CH3OH(g) + 3 O2(g) 2 CO2(g) + 4 H2O(g) (a) Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for this reaction. What is the enthalpy of combustion of one mole of gaseous methanol? (b) Compare your answer in part (a) with the value of tHcalculated using enthalpies of formation data.arrow_forward
- Ethanol can be made by the reaction of ethylene and water: H2C=CH2(g) + H2O(g) CH3CH2OH(g) Use bond dissociation enthalpies to estimate the enthalpy change in this reaction. Compare the value obtained to the value calculated from enthalpies of formation.arrow_forwardA common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete Lewis structure for an organic compound called methyl cyanoacrylate, the main ingredient in Super Glue. Draw a complete Lewis structure for methyl cyanoacrylate in which all atoms have a formal charge of zero.arrow_forwardFructose, C6H1206(S), consists of 5 C-C single bonds, 7 C-O bonds, 7 C-H bonds, and 5 O-H bonds with average bond energies of 348 kJ/mol, 360 kJ/mol, 412 kJ/mol, and 463 kJ/mol respectively. The bond energy for C=O is 799 kJ/mol and O=O is 498 kJ/mol. The molar mass of fructose is 180.12 g/mol. Estimate the change in enthalpy if 2.56 g of fructose undergoes complete combustion at standard temperature and pressure.arrow_forward
- ) Cars with internal combustion engines burn gasoline (octane, C8H18) but analternative electric vehicle burns hydrogen gas to generate electricity. Although gasoline is amixture of hydrocarbons, it can be represented as consisting only of octane, C8H18.a. Write the equation for the combustion of hydrogen gas. This is a synthesis (combination)reaction with water vapor as the product.b. Draw Lewis structures for all molecules in the combustion of hydrogen equation..c. Use bond energies and Lewis structures to calculate the energy released in thecombustion of hydrogen. Use the bond energies table HERE . You can also find thisinformation in week 9, Wk9_Energy_Stoichiometry_notes .d. Which fuel, octane or hydrogen, has a higher fuel value? Octane has a fuel value of5140 kJ/mol. Express the fuel value in kJ/g.e. Calculate the amount of energy released when 400.0 g of hydrogen are combustedarrow_forwardHydrogen azide (HN3) is a shock-sensitive liquid, which means it explodes when subjected to a physical shock. The HN3molecule contains two N-N bonds with bond lengths 113 pm and 124 pm. The H-N-N bond angle is 112°. Draw two Lewis structures of HN3 that obey the octet rule. What is the formal charge of each atom in your structures? Which structure is most consistent with the experimental data?arrow_forwardAcetylene (C2H2) and nitrogen (N2) both contain a triplebond, but they differ greatly in their chemical properties.(a) Write the Lewis structures for the two substances. (b) By referring to Appendix C, look up the enthalpies of formationof acetylene and nitrogen. Which compound is more stable?(c) Write balanced chemical equations for the completeoxidation of N2 to form N2O5(g) and of acetylene to formCO2(g) and H2O(g). (d) Calculate the enthalpy of oxidationper mole for N2 and for C2H2 (the enthalpy of formationof N2O5(g) is 11.30 kJ/mol). (e) Both N2 and C2H2 possesstriple bonds with quite high bond enthalpies (Table 8.3).Calculate the enthalpy of hydrogenation per mole for bothcompounds: acetylene plus H2 to make methane, CH4;nitrogen plus H2 to make ammonia, NH3.arrow_forward
- Hydrazine, N2H4, burns in oxygen as follows: N2H4 + O2 → N2 + 2H2O [The bond energies in kJ/mol are: N-H = 388; N-N 163; N≡N 944; O-H 463; O=O 496] Draw the chemical structures of the reactants and products and give the formula to calculate enthalpy change in a reaction, ΔH.arrow_forwardDraw the Lewis structure for the polyatomic amide (NH)) anion. Be sure to include all resonance structures that satisfy the octet rule. X 500: [] ?arrow_forwardH. H. H. H. H. The diagram above shows two resonance structures for a molecule of C6H6. The phenomenon shown in the diagram best supports which of the following claims about the bonding in C6H6 ? (A) In the C6H6 molecule, all the bonds between the carbon atoms have the same length. (B) Because of variable bonding between its carbon atoms, C6H6 is a good conductor of electricity. (C) The bonds between carbon atoms in C6H6 are unstable, and the compound decomposes quickly. The C6H6 molecule contains three single bonds between carbon atoms and three double bonds between (D) carbon atoms. IIUarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





