
Concept explainers
(a)
Interpretation:
Appropriate products are to be drawn for the given proton transfer reaction with curved arrow notation.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two valence electrons is shown by curved arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
Answer to Problem 6.38P
For the given proton transfer reaction, the products are as shown below.
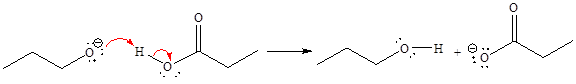
Explanation of Solution
The curved arrow notation for the given proton transfer reaction is shown below:
In a proton transfer reaction, a proton is transferred from the Bronsted-Lowry acid to the Bronsted-Lowry base. The movement of two valence electrons is shown by curved arrow. The head of the arrow pointing to the atom shows the transfer of valence electrons to form a new single bond. In the above reaction, one curved arrow is drawn from the lone pair on O to the H on the other reactant, so it illustrates the formation of a new
The products of the given proton transfer reaction are drawn on the basis of the curved arrow notation that shows breaking and formation of bonds.
(b)
Interpretation:
Appropriate products are to be drawn for the given proton transfer reaction with curved arrow notation.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two valence electrons is shown by curved arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
Answer to Problem 6.38P
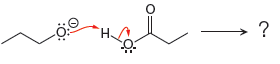
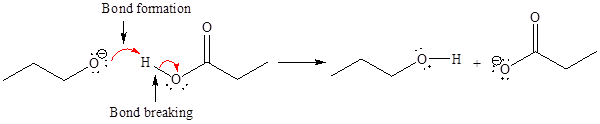
For the given proton transfer reaction the products are drawn as below.
Explanation of Solution
The curved arrow notation for the given proton transfer reaction is shown below:
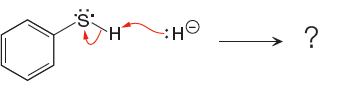
In a proton transfer reaction, a proton is transferred from the Bronsted-Lowry acid to the Bronsted-Lowry base. The movement of two valence electrons is shown by curved arrow. The head of the arrow pointing to atom shows the transfer of valence electrons to form a new single bond. In the above reaction, one curved arrow is drawn from the lone pair on H to the H of the first reactant, so it illustrates the formation of a new
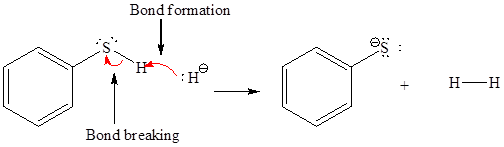
The products of the given proton transfer reaction are drawn on the basis of the curved arrow notation that shows breaking and formation of bonds.
(c)
Interpretation:
Appropriate products are to be drawn for the given proton transfer reaction with curved arrow notation.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two valence electrons is shown by curved arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
Answer to Problem 6.38P
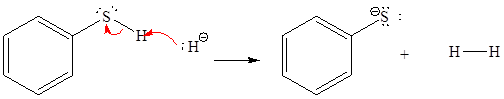
For the given proton transfer reaction, the products are drawn as below.
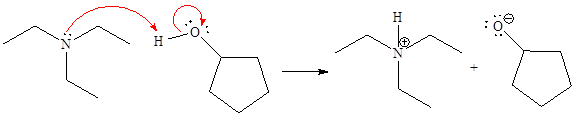
Explanation of Solution
The curved arrow notation for the given proton transfer reaction is shown below:
In a proton transfer reaction, a proton is transferred from the Bronsted-Lowry acid to the Bronsted-Lowry base. The movement of two valence electrons is shown by curved arrow. The head of the arrow pointing to an atom shows the transfer of valence electrons to form a new single bond. In the above reaction, one curved arrow is drawn from the lone pair on N to the H of the second reactant, so it illustrates the formation of a new
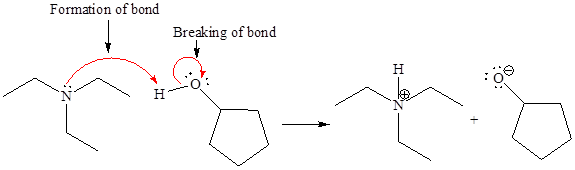
The products of the given proton transfer reaction are drawn on the basis of the curved arrow notation that shows breaking and formation of bonds.
(d)
Interpretation:
Appropriate products are to be drawn for the given proton transfer reaction with curved arrow notation.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two valence electrons is shown by curved arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
bond. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton.
Answer to Problem 6.38P
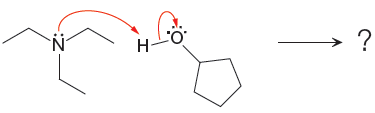
For the given proton transfer reaction, the products are drawn as below.
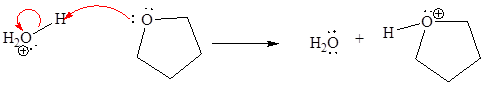
Explanation of Solution
The curved arrow notation for the given proton transfer reaction are shown below:
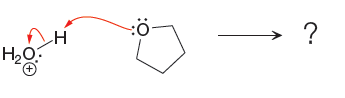
In a proton transfer reaction, a proton is transferred from the Bronsted-Lowry acid to the Bronsted-Lowry base. The movement of two valence electrons is shown by curved arrow. The head of the arrow pointing to an atom shows the transfer of valence electrons to form a new single bond. In the above reaction, one curved arrow is drawn from the lone pair on O to the H of the first reactant, so it illustrates the formation of a new
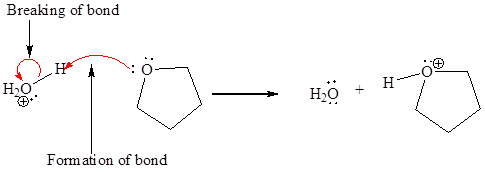
The products of the given proton transfer reaction are drawn on the basis of the curved arrow notation that shows breaking and formation of bonds.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Add the curved arrow notation to this proton transfer reaction. Then classify each starting material according to its reactivity role in the reaction. ++ -Ö-Harrow_forwardFollow the format of solving the problem where you should write the GIVEN, ASKED, SOLUTION, and ANSWER. Add curved arrows to the following reactions to indicate the flow of electrons for all of the bond-forming and bond-breaking steps.arrow_forwardGiven the curved arrow notation for each of the following proton transfer reactions, draw the appropriate products. (a) (b) ? OH: (c) (d) H- ? ? :0arrow_forward
- question in image below please show all steps needed.arrow_forwardIn this reaction, is HCL can be considered as a by product? If yes please explain and draw the reactionarrow_forwardPlease draw arrow-pushing reaction mechanism and identify number of electrons involved here. This is an electrolytic reactionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
