
Concept explainers
(a)
Show the direction
(a)
Explanation of Solution
Show the direction vector in the FCC unit cell for the cubic direction
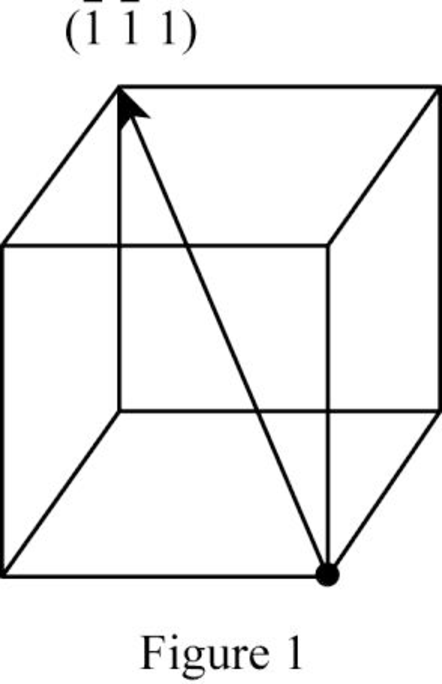
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(b)
Show the direction vector in a FCC unit cell for the cubic direction
(b)
Explanation of Solution
Show the direction vector in the FCC unit cell for the cubic direction
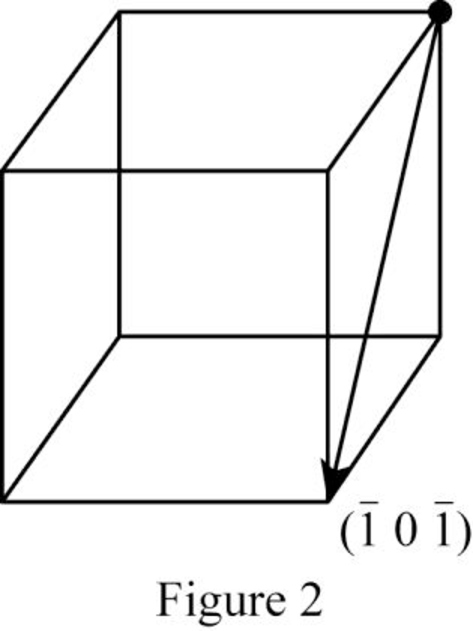
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(c)
Show the direction vector in a FCC unit cell for the cubic direction
(c)
Explanation of Solution
Show the direction vector in the FCC unit cell for the cubic direction
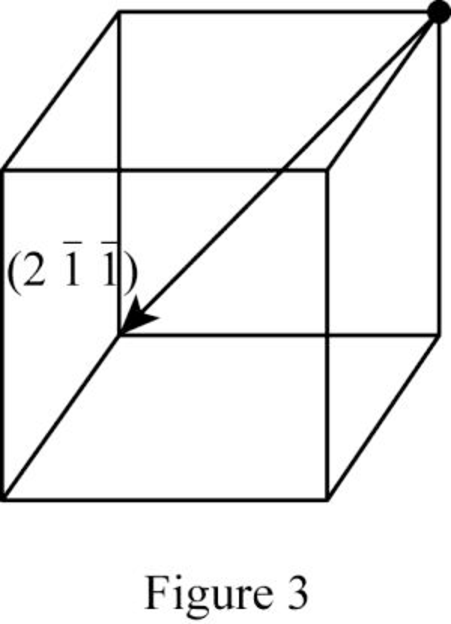
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(d)
Show the direction vector in a FCC unit cell for the cubic direction
(d)
Explanation of Solution
Show the direction vector in the FCC unit cell for the cubic direction
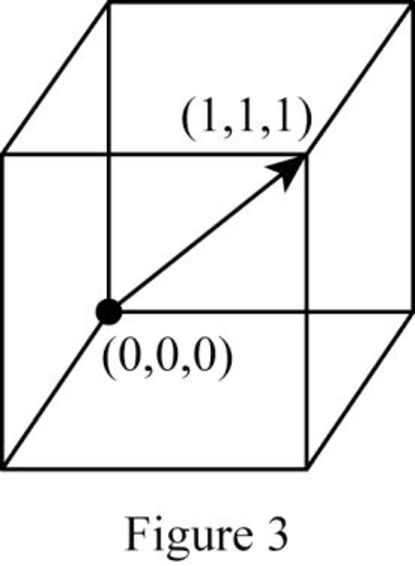
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(e)
The angle between the direction vectors
(e)
Answer to Problem 30AAP
The angle between the direction vectors
Explanation of Solution
Write the expression to calculate angle between the direction vectors
Here, Miller indices of the cubic plane 1 are
Conclusion:
Substitute 1 for
Thus, the angle between the direction vectors
Want to see more full solutions like this?
Chapter 3 Solutions
Foundations of Materials Science and Engineering
- a: Sketch within a cubic unit cell the following crystallographic directions: [121], [201], [2 1 3), [111] [121] hi Dats JD fallarrow_forward(b) Draw the following direction vectors in a cubic unit cell: [212] and [101] Draw the following crystallographic planes in a cubic unit cell: (111) and (121) "oin 2/2 Page 1 of 2arrow_forwardDraw the following direction vectors in an orthorhombic unitcell:[111],[201]and [2 ̄31]. with lattice parameters {1, 2, 3, 90, 90, 90}arrow_forward
- Determine the angles a, ß, and y that are listed in the cubic unit cell provided. Enter the angles in degrees. Note: You should be able to use basic facts about cube geometry and crystallographic convention to solve this, rather than elaborate direction cosine equations. [00 1] (0 0 1) [1O 1] [11 1) (1 1 1) a = i B = iarrow_forward1. Within a cubic unit cell, sketch the following directions: [110], [121], [0Ī2], [123], [103]arrow_forwardHelp me pleasearrow_forward
- Within a cubic unit cell, sketch the following directions: (a) [110] (b) [121] (c) [012] (d) [133]arrow_forward1. Many substances crystallize in a cubic structure. The unit cell for such crystals is a cube having an edge with a length equal to do. -Face diagonal a. What is the length, in terms of do, of the face diagonal, which runs diagonally across one face of the cube? (Hint: Use the Pythagorean theorem.) b. What is the length, again in terms of do. of the cube diagonal, which runs from one corner, through the center of the cube, to the opposite corner? (Hint: Make a right triangle having a face diagonal and an edge of the cube as its sides, with the hypotenuse equal to the cube diagonal, then use the Pythagorean theorem again.) 2. In an FCC structure, the centers of the atoms are found on the corners of the cubic unit cell and at the center of each face. The unit cell has an edge whose length is the distance from the center of one corner atom to the center of another corner atom on the same edge. The atoms on the diagonal of any face are touching. One of the faces of the unit cell is shown…arrow_forwardB) Draw the following planes in the hexagonal crystal: (1) (1 0i ) (2) (ī010) (3) ( 0ī 11) (4) ( 0 1 o) C) Draw the following direction vectors in the cubic unit cell: (1) [111] (2) [011] (3) [110] (4) [112]arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





