
To review:
The types of forces, which help the insulin to bind to its target, and the truth about the amino acids placed at positions B23, B24, A2, A19, and A3, which allow them to get involved in the substrate binding.
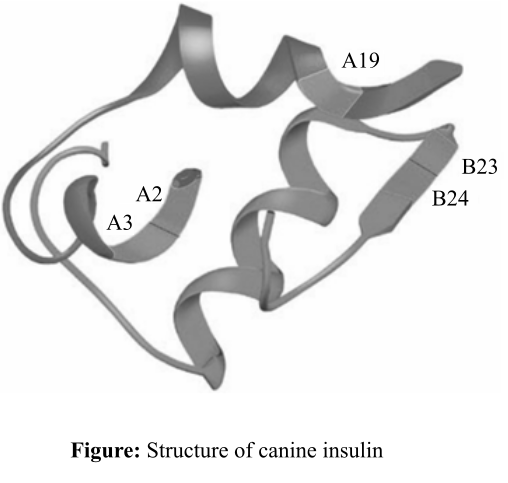
Figure: Structure of canine insulin.
Introduction:
The quaternary structure of the proteins involves two or more chains of the peptides that held together with the help of various covalent and noncovalent forces like hydrogen bonds, van der Waal forces of attraction, and disulfide linkages. An enzyme is held to its substrate with the help of van der Waal forces of attraction.
Explanation of Solution
Insulin is an enzyme, which helps to lower the blood glucose level by storing it in the form of glycogen in the muscle tissues. It binds to its substrate, that is, glucose by the noncovalent van der Waal interactions. The van der Waal interactions occur over a short distance and are weak forces. Generally, enzymes bind to their substrate with the help of these forces so that products formed could be detached easily from the enzyme and it again becomes available for the next cycle of reaction.
The part of the insulin enzyme, that is involved in van der Waal interaction, contains hydrophobic amino acid groups (−R group), for example, valine, isoleucine, glycine, and phenylalanine, which might have placed on the positions such as A2, A3, A19, B23, and B24. The –R groups protrude out of the insulin side chain so that they could interact with the target molecules or the amino acids that are present in the substrates.
Thus, it can be concluded that the noncovalent interactions like van der Waal forces help the insulin to bind to its substrates. Amino acids at the positions B23, B24, A2, A19, and A3 might contain hydrophobic side chain amino acid groups so that they help insulin to bind to substrates.
Want to see more full solutions like this?
- By what the two polypeptides of human insulin are linked together?arrow_forwardIf both cysteine residues on the B chain of insulin were changes to alanine residues, how would it affect the quaternary structure of insulin?arrow_forward64 An investigator is studying the mechanism of action of an insulin-sensitizing drug. The addition of the drug to cultured mouse hepatocytes is observed, and results show increased phosphorylation and inhibition of acetyl-CoA carboxylase Which of the following processes is most likely inhibited by this action of the drug? A) Fatty acid oxidation B) Fatty acid synthesis C) Glycogen synthesis D) Glycolysis E) Protein synthesisarrow_forward
- What is the product of CAMP phosphodiesterase activity?arrow_forwardWhy are the essential fatty acid associated with low incidence of heart disease? Cite some clinical signs of essential fatty acid deficiency. Explain how aspirin can block the synthesis of prostaglandins?arrow_forwardThe hormones insulin and glucagon play an important role in the regulation of plasma glucose.a) Discuss the antagonistic actions of the hormones insulin and glucagon in regulating blood glucose levels within a narrow physiological range.arrow_forward
- What does this important observation imply about the relation between the amino acid sequence of insulin and its three-dimensional structure?arrow_forwardHbA1c is used to monitor blood glucose levels because hemoglobin is the only protein in blood that is covalently modified by glucose. True False Insulin Glargine is a long-acting form of insulin that is synthesized with several D-amino acids that slow its proteolytic degradation and extend the half-life of the insulin Glargine molecule. True Falsearrow_forwardWhat is the raw material used for human insulin produced using E.coli? What is the stoichiometric equation of insulin production?arrow_forward
- What is glucagon-like peptide-1 (GLP-1) ?arrow_forwardWhat is an insulinarrow_forwardBodybuilders use a variety of anabolic substances to gain mass. Two such compounds are insulin and trenbolone. Insulin has significant anabolic and anti-catabolic properties and impacts the metabolism of various macromolecules, not just that of carbohydrates. Trenbolone binds the androgen receptor with an affinity five times higher than that of testosterone and is popular for its fat-burning and anabolic properties. H. Trenbolone C18H2202 Insulin C257H383NosO77S6 How do the different targets, mechanisms of action and durations of each drug ultimately lead to the same desired effect (increase in lean body mass)?arrow_forward
