
(a)
Interpretation: The formula of conjugate base of sulfurous acid should be written.
Concept introduction:In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
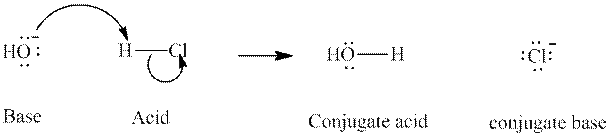
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(b)
Interpretation: The formula of conjugate base of chloric acid should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
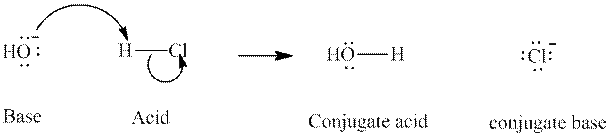
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(c)
Interpretation: The formula of conjugate base of hydrogen sulphide should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
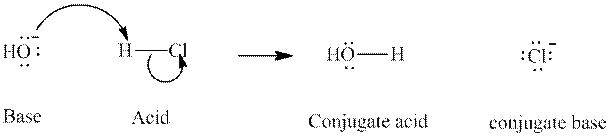
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(d)
Interpretation: The formula of conjugate base of dimethyloxonium should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
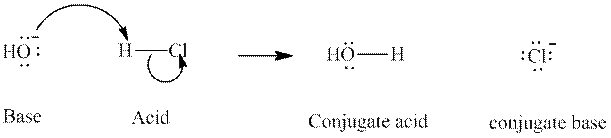
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(e)
Interpretation: The formula of conjugate base of hydrogen sulphate should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
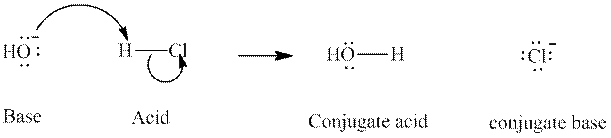
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- Chemists working with fluorine and its compounds some- times find it helpful to think in terms of acid-base reac- tions in which the fluoride ion (F¯) is donated and ассеpted. (a) Would the acid in this system be the fluoride donor or fluoride acceptor? (b) Identify the acid and base in each of these reactions: CIF;O2 + BF; CIF,O, · BF, -- TiF, + 2 KF – K2[TiF,]arrow_forwardThe following two reactions involve proton transfer. In each reaction, identify the acid and the base. Identify the conjugate base of the acid, and the conjugate acid of the base. Draw Lewis electron dot diagrams for each molecule, and describe the rear- rangement of bonding in the reaction as nonbonding electron pairs become cova- lently shared pairs and vice versa. (a) H¿O(€) + NH (aq) → H;0* (aq) + NH3(aq) (b) CH;CH;OH(aq) + NH7 (aq) → CH;CH;O¯(aq) + NH3(aq)arrow_forwardWhat is the pH of a 0.10 M N(CH3)3(aq)? The base ionization constant of trimethylamine is Kb= 6.5×10–5. Enter your answer with correct units and significant figures.arrow_forward
- The pH of an aqueous solution of 1.08×10-2 M carbonic acid, H2CO3 (aq), isarrow_forwardResearchers working with glasses often think of acid-base reactions in terms of oxide donors and oxide acceptors. The oxide ion is O2-. (a) In this system, is the base the oxide donor or the oxide ассeptor? (b) Identify the acid and base in each of these reactions: 2 CaO + SiO2 Ca,SiO4 Сa, SiO, + SiOz —2 СaSiO, | Сa, SiO, + Ca0 — СазSiO;arrow_forwardSodium hydroxide is a strong electrolyte and hydrates in water according to the following equation: NaOH (s) → Na* (aq) + OH (aq) The concentration of NaOH is 6.66 x 103, Calculate the pH of the solution.arrow_forward
- The pH of an aqueous solution of 0.1690 M hydrosulfuric acid, H₂S (aq), isarrow_forwardWhat volume of 0.250 M HCl(aq) will completely react with 50.0 mL of 0.115 M NaOH(aq)? Write the balanced reaction and show the stoichiometry. Write the reaction of acetate ion in water. Predict the approximate pH of a solution with acetate ion present.arrow_forwardWhat is the pH of a 1.00 molar solution of NH4Cl(aq)? The Kb for NH3 = 1.8 × 10–5 .arrow_forward
- Calculate pH of 2.5 x 10-2 M NaOH(aq). NaOH(aq) is strong base.arrow_forwardIn a solution of 0.015 mol L-1 HBr(aq) at 25 °C (a) What are the concentrations of the hydronium, H3O+, and hydroxide, OH-, ions? (b) What is the pH of the solution?arrow_forwardIf weak acids ionize only a few percent in aqueous solution, why is it possible to fully neutralize a weak acid by reacting it with the stoichiometric equivalent of sodium hydroxide solution, NaOH(aq)?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


