
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 9E
These are NOTlegitimate Lewisstructures (and aremissing formalcharges). Show (as inthe example) how apair of electrons canbe moved to make theLewis structurelegitimate.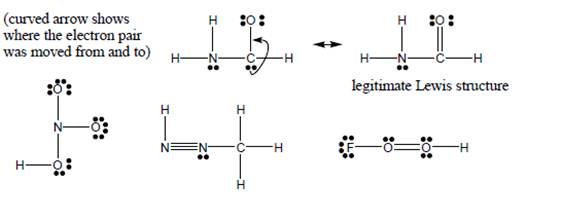
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
D Lewis strct
molecalar iodie, and the dide in
and uith notatich.
5.Assuming that C and N have similar
electronégativities raw te mdeular orbital
diagram
valence orbitals
Include onty tle
s for (N and CN Include onty the
and label all tb atonic
pund
Pub
Standakd
hotatich.
Also, label the moecular orebitals that are
HOMO and LUMO, D.o these mclecults
stable state Cexplain 1\Jour
wn S 910 101 o
the
し
9. The following figure shows the potential energy as a function of the distance between two
hydrogen atoms. (a) Label the axes (include reasonable units!) and indicate the equilibrium bond
distance. (b) What is the energy when the atoms are infinitely far apart? (c) On the same plot,
sketch what you think the potential energy of the anion H; might look like. Consider where you
would put the equilibrium bond distance and how deep the potential energy dips down.
Chapter 2 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 2 - Prob. 1CTQCh. 2 - The valence shell of an atom in a legitimate Lewis...Ch. 2 - Prob. 3CTQCh. 2 - Prob. 4CTQCh. 2 - Prob. 5CTQCh. 2 - It is impossible to draw a legitimate Lewis...Ch. 2 - Describe how to calculate the total number of...Ch. 2 - Prob. 8CTQCh. 2 - Prob. 9CTQCh. 2 - Prob. 10CTQ
Ch. 2 - Prob. 11CTQCh. 2 - Prob. 12CTQCh. 2 - A complete Lewis structure must show all nonzero...Ch. 2 - Prob. 14CTQCh. 2 - Prob. 15CTQCh. 2 - Prob. 16CTQCh. 2 - Prob. 17CTQCh. 2 - Prob. 18CTQCh. 2 - Complete the rest of the table for N, O or X by...Ch. 2 - Prob. 20CTQCh. 2 - Prob. 21CTQCh. 2 - Make a checklist that can be used to determine if...Ch. 2 - Prob. 2ECh. 2 - Prob. 3ECh. 2 - Draw the Lewis structure of a neutral molecule...Ch. 2 - Prob. 5ECh. 2 - For each element, predict (and draw a Lewis...Ch. 2 - Predict which of the following species is least...Ch. 2 - The molecules BH3 and SF6 and the ion SO42 exist...Ch. 2 - These are NOTlegitimate Lewisstructures (and...Ch. 2 - Fill in missing formal charges where needed (all...Ch. 2 - Below each structure in the previous question is a...Ch. 2 - Prob. 12ECh. 2 - Carbon monoxide (CO) is an example of an overall...Ch. 2 - Explain why this Lewis structure for CO is not as...Ch. 2 - Prob. 15ECh. 2 - Prob. 16ECh. 2 - Prob. 17ECh. 2 - Prob. 18ECh. 2 - Prob. 19E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Predict the geometry of the SbCl52 ion, using the VSEPR method. (b) The ion SbCl63 is prepared from SbCl52 by treatment with Cl . Determine the steric number of the central antimony atom in this ion, and discuss the extension of the VSEPR theory that would be needed for the prediction of its molecular geometry.arrow_forwardThe structure of pyridine is When a proton becomes bonded to the nitrogen atom by way of its unshared electron pair, the result is _____________________________arrow_forwardHow many orbitals are allowed in asub shell if 2 B5 An organic flavoring agent extraoted fram A PePPer mint oil.it contains c, Hr and o. in o ne combus tion amlysis) 0:0100g of the Substance yields 0.01153 g H,o and 0,02816g co . what is the empirical formula and mdecular formula of this f lvorng agent is the molar mass is 156l 9 Imolarrow_forward
- Use the information on the fictious nonmetal elements. to answer the following Element Colomn EN ff 1.9 14 17 3.1 2.3 3.6 15 Nn 16 Shoos one of the followng mokcular compounds and completc the queSTIons: Nn Oyzlshape is benti V) ODraw a leuis Structure Shoudng all lalonce electrons. ) Calculate the electrongativity the bond. State what a paar covalert band, notate the partial difference for Gf bond will form If it is charges d) Detamine if the molecule is polar or polar. non-arrow_forwardDraw the Lewis structures for each of the following moleculesor ions. Identify instances where the octet rule is notobeyed; state which atom in each compound does not followthe octet rule; and state how many electrons surround theseatoms: (a) NO, (b) BF3, (c) ICl2-, (d) OPBr3 (the P is the centralatom), (e) XeF4.arrow_forward10. The following Lewis structures for (a) HCN, (b) C3H;, (c) SnOz, (d) BF3, (e)HOF are incorrect. Explain what is wrong with each one and give a correct structure for the molecule. (Relative positions of atoms are shown correctly.) (a) H-ëN (b) HCC-H (c) 0-Sn-0 (d) :F B :F: (e) H-O-F:arrow_forward
- (a) Write a single Lewis structure for SO3, and determine thehybridization at the S atom. (b) Are there other equivalentLewis structures for the molecule? (c) Would you expect SO3to exhibit delocalized p bonding?arrow_forwardWhich is the more polar bond in each of the following pairsfrom: (a) Br--Cl; (b) F--Cl; (c) H--O; (d) Se--H; (e) As--H; (f) S--N. (a) or (b); (c) or (d); (e) or (f)?arrow_forwardDraw the Lewis structures for each of the following ionsor molecules. Identify those in which the octet rule is notobeyed; state which atom in each compound does not followthe octet rule; and state, for those atoms, how manyelectrons surround these atoms: (a) PH3, (b) AlH3, (c) N3-,(d) CH2Cl2, (e) SnF62-.arrow_forward
- please both explain otherwise dontarrow_forwardTlatch chemtcal terminelogy vzittecorrect definitiom is a measure of the tendency of an atom to A EectronegatVIEY attrart electrone BValence electrons Is used to show the bonding beween atome of a CEone nar motecute and the Ione pairs of eloctrons that may exist in the imolecule Bond that farms hetveen izo atoms where the electrons are unoqually distributed causing the molecuile to have a 3light electrieal dioole moment is called DLewis structure ENarpolar bond Bond that formIs between we atoms where the siecttons ate oqually distributed is caltad v Valerice elaconsthat ar notsharec wtaianuther atom are caled hathe Eltons l tha Stiferimarrow_forwardWhat are the formal charges on the atoms in the following Lewis structure for HNO3? 1 2 4 :ö-N-Ö-H O 1; 0; 1; 0; -2 0; 0; 0; 0; 0 O 0; 0; 0; 1; -1 O 1; 0; 0; 0; -1 0; 0; 1; 0; -1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY