
Concept explainers
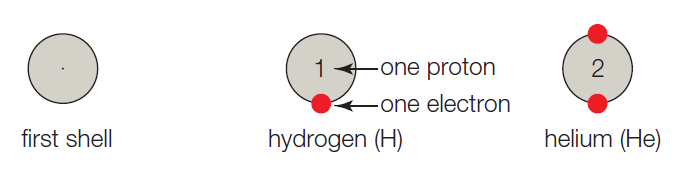
- A. The first shell corresponds to the first energy level, and it can hold up to 2 electrons. Hydrogen has one proton, so it has 1 electron and one vacancy. A helium atom has 2 protons, 2 electrons, and no vacancies.
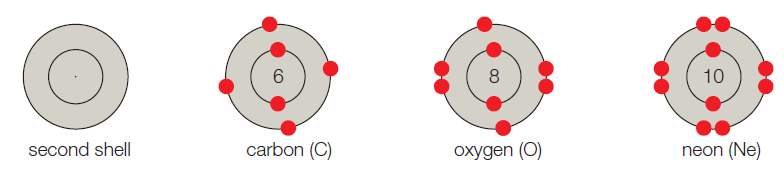
- B. The second shell corresponds to the second energy level, and it can hold up to 8 electrons. Carbon has 6 electrons, so its first shell is full. Its second shell has 4 electrons and four vacancies. Oxygen has 8 electrons and two vacancies. Neon has 10 electrons and no vacancies.
- C. The third shell corresponds to the third energy level, and it can hold up to 8 electrons. A sodium atom has 11 electrons, so its first two shells are full; the third shell has one electron. Thus, sodium has seven vacancies. Chlorine has 17 electrons and one vacancy. Argon has 18 electrons and no vacancies.
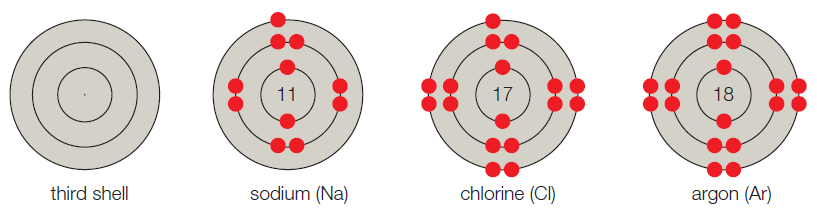
Figure It Out: Which of these models have unpaired electrons in their outer shell?
The atoms are referred to as building blocks of all substances. In other words, it is the basic fundamental unit of the matter. In every atom, the uncharged neutron and positively charged protons are present in the nucleus whereas the negatively charged electron moves around the nucleus. Generally, the shell model system is used to study the valence status of an atom. The atomic number is the number of protons present in the atom which determines the type of the atom
Explanation of Solution
The given shell model consists of about eight atoms namely helium, neon, hydrogen, oxygen, carbon, sodium, chloride, and argon. Of this given atom in the model the hydrogen, carbon, oxygen, chlorine and sodium have unpaired electrons in the outer shell. These unpaired electrons are available to pair with other unpaired electrons in another atom. Thus these atoms combine to form molecules.
Want to see more full solutions like this?
Chapter 2 Solutions
Biology Today and Tomorrow without Physiology (MindTap Course List)
- Which of the following statements is a lie? Select one: a. Chemical energy is the energy within chemical bonds. Energy is required for bonds to break while energy is released when bonds form. b. Exergonic chemical reactions release more energy than they absorb and the catabolism of the food in an energy bar serves as an example. c. The many forms of energy used up by cells comes from the breakdown of the chemical energy from the food we digest.arrow_forwardOxygen (O) is a(n) _______; the oxygen we breathe (O2) is a(n) _______; and the carbon dioxide we exhale is a(n) _______. a. compound; molecule; element b. atom; compound; element c. element; atom; molecule d. atom; element; molecule e. element; molecule; compoundarrow_forwardMagnesium has an atomic number of 12. Which of the following statements is true of a neutral magnesium atom? a. It has 12 protons, 12 electrons, and 12 neutrons. b. It has 12 protons, 12 electrons, and six neutrons. c. It has six protons, six electrons, and no neutrons. d. It has six protons, six electrons, and six neutrons.arrow_forward
- The pH of lemon juice is about 2.0, whereas tomato juice’s pH is about 4.0. Approximately how much of an increase in hydrogen ion concentration is there between tomato juice and lemon juice? a. 2 times b. 10 times c. 100 times d. 1000 timesarrow_forwardA molecule that donates electrons becomes _________, and the one that accepts the electrons becomes ________ . a. reduced; oxidized c. oxidized; reduced b. ionic; electrified d. electrified; ionicarrow_forwardThis class of organic molecules contains important fuel molecules A. Carbohydrates as well as provide structure and energy storage. This class of organic molecules contains important fuel molecules A. Carbohydrates as well as provide structure and energy storage. Proteins Carbohydrates Lipids Nucleic acidsarrow_forward
- Identify all of the elements in the paragraph above. Use your periodic table along with what you learned in live lesson to make your own chart of those elements when they are oxidized..arrow_forwardIsotopes differ from each other only in the number of electrons they contain. Select one: a. True b. Falsearrow_forwardChoose the best answer for following question. An atom that has two electrons in the valence shell, such asmagnesium, would most likelya. share to acquire a completed outer shell.b. lose these two electrons and become a negatively charged ion.c. lose these two electrons and become a positively charged ion.d. bind with carbon by way of hydrogen bonds.e. bind with another magnesium atom to satisfy its energy needs.arrow_forward
- 1. a. What is the abbreviated name for the molecule below? (3 letters) What is the abbreviated name of this molecule if it has two phosphates? What is the abbreviated name of this molecule if it has one phosphate? From your answers above, circle the form of the molecule that has the most energy. What type of energy is this? circle all that apply: (KINETIC / POTENTIAL / CHEMICAL) Where is the energy? b. Identify the three main parts of this molecule. (write on the red brackets i-iii) c. Parts ii and ii together make (blue vertical bracket) ii. NH2 НО-Р ОН ОН ОН ОН ОН iii d. What is the specific name for this molecule? e. This molecule is also a monomer building block of which biomolecule (be specific) The general name for this type of monomer is 2. a. Put arrows by the two high energy bonds on the molecule above. Explain why these functional groups are difficult to join. (hint: these are acids. Circle the H that will be donated at cellular pH). (MORE / LESS/ the SAME amount of) energy…arrow_forwardOn the energy diagram below, which point represents a transition state? energy A B. Point 1 Point 2 C. Point 3 D. Point 4 reaction coordinatearrow_forwardNow imagine if you removed one hydrogen from methane and added a second carbon and its associated hydrogens. This would create ethane. Draw your completed ethane molecule below (ball and stick model).arrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning



