
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 11P
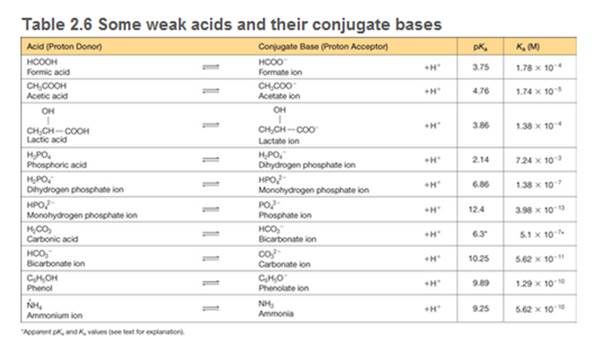
You need to make a buffer whose pH is 7.0, and you can choose from the weak acids shown in Table 2.6. Briefly explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
amino glycine pka is 2.4, 9.8 Calculate the
most effective buffering range.
if there is two pka, since there are 2 buffer
region, is the buffering range 1.4~3.4 and
8.8~10.8?
i would like to know the correct answer and
detailed solution/reason.
If I want to make a 100mL buffer that has 2% SDS, 100mM tris pH 8, 10mM EDTA, and proteinase K [1ug/ml]; How much of each would I need to add to create the buffer?
TE buffer consists of 10 mM of Tris-Cl, pH 7.6 and 1mM of EDTA, pH 8.0. You need to prepare 3L of TE buffer and you have the following stock solutions: 500 mM Tris-Cl and 1 M EDTA. How will you prepare the 3L of TE? Write work clearly
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Buffers also exist in biological systems. Discuss the composition and function of one example of a biological buffer.arrow_forwardIt is possible to make a buffer that functions well near pH7 using citric acid, which contains only carboxylate groups. Explain.arrow_forwardHow do you prepare 500 mL pH 6 Citric acid/Phosphate buffer in the laboratory. Please explain briefly.arrow_forward
- Describe how you would prepare a 500 ml of 0.35M Glycine pH2.2 from a 5M Glycine solution of pH 7. Show your calculations.arrow_forwardI want to make a buffer solution containing 1 M glycene pH = 10 (Na+), 1 mM ZnCl2 , 1 mM MgCl2. I have the stock solutions 1 M glycene, 1 M MgCl2, 1 M ZnCl2, and 10 M NaOH. My final volume of buffer solution needs to be 250ml. how much (in ml) of each solution should i put into my buffer?arrow_forwardYou have a 100X stock of a buffer. You need 100 ml of 1X buffer. How much buffer solution do you use? How much water?arrow_forward
- If 4 mL of 1 M NaOH is added to 100 mL buffer, would it still be a usable buffer according to the conventions? Explain why or why not.arrow_forwardYou want to prepare a 15 mL gel containing 0.8% (weight /volume) agarose. How much agarosedo you need to weigh to prepare the solution? Show all your calculations.Hint: Write down the units for 0.8% knowing that agarose is a powder that is mixed with aliquidarrow_forwardYou need to prepare an acetate buffer of pH 5.43 from a 0.621 M acetic acid solution and a 2.95 M KOH solution. If you have 730 mL of the acetic acid solution, how many milliliters of the KOH solution do you need to add to make a buffer of pH 5.43? The pKa of acetic acid is 4.76. Be sure to use appropriate significant figures.arrow_forward
- how to prepare a 100 mL of 4x sds buffer from a 1.0 L of 50x SDS stock solution. Show complete solutionarrow_forwardHow much of the enzyme proteinase k (solute) is required to make 250ml of a solution with a concentration of .01mg/ml? (Weight/volume ratio)arrow_forwardYou need to make a protein buffer of: . • • 100 mM NaCl 25 mM Tris 8 5% w/v glycerol • 2 mM DTT Your stock solutions are: 5 M NaCl • 2 M Tris 8 • 70% w/v glycerol • DTT @ 154.25 g/mol How would you make a 1L protein buffer Solution? Show your work and describe.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license