
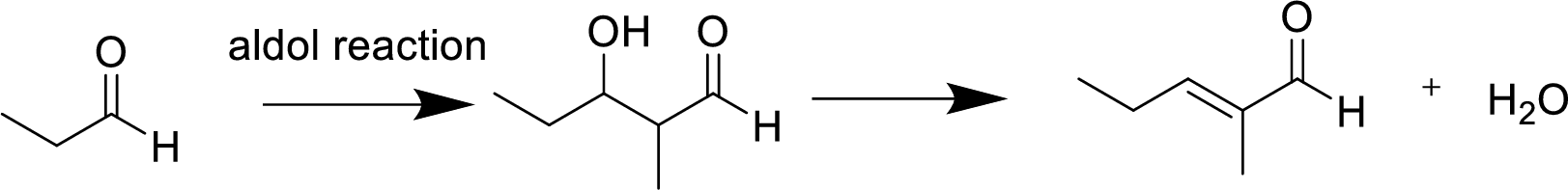
(a)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of
(a)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
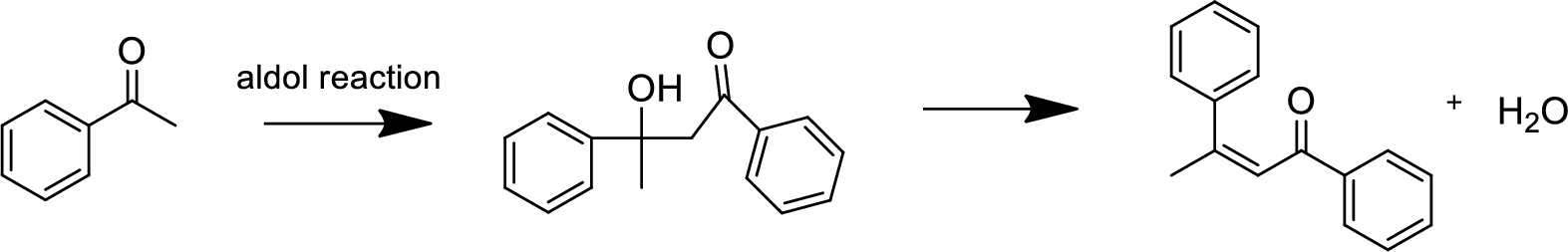
(b)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of aldehydes and ketones. Aldol reaction is a reversible reaction and occurs in the presence of a strong base like sodium hydroxide. One molecule (aldehyde or ketone) acts a nucleophile and attacks the electrophilic carbon center of the other molecule to give the addition product. The product is named
(b)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
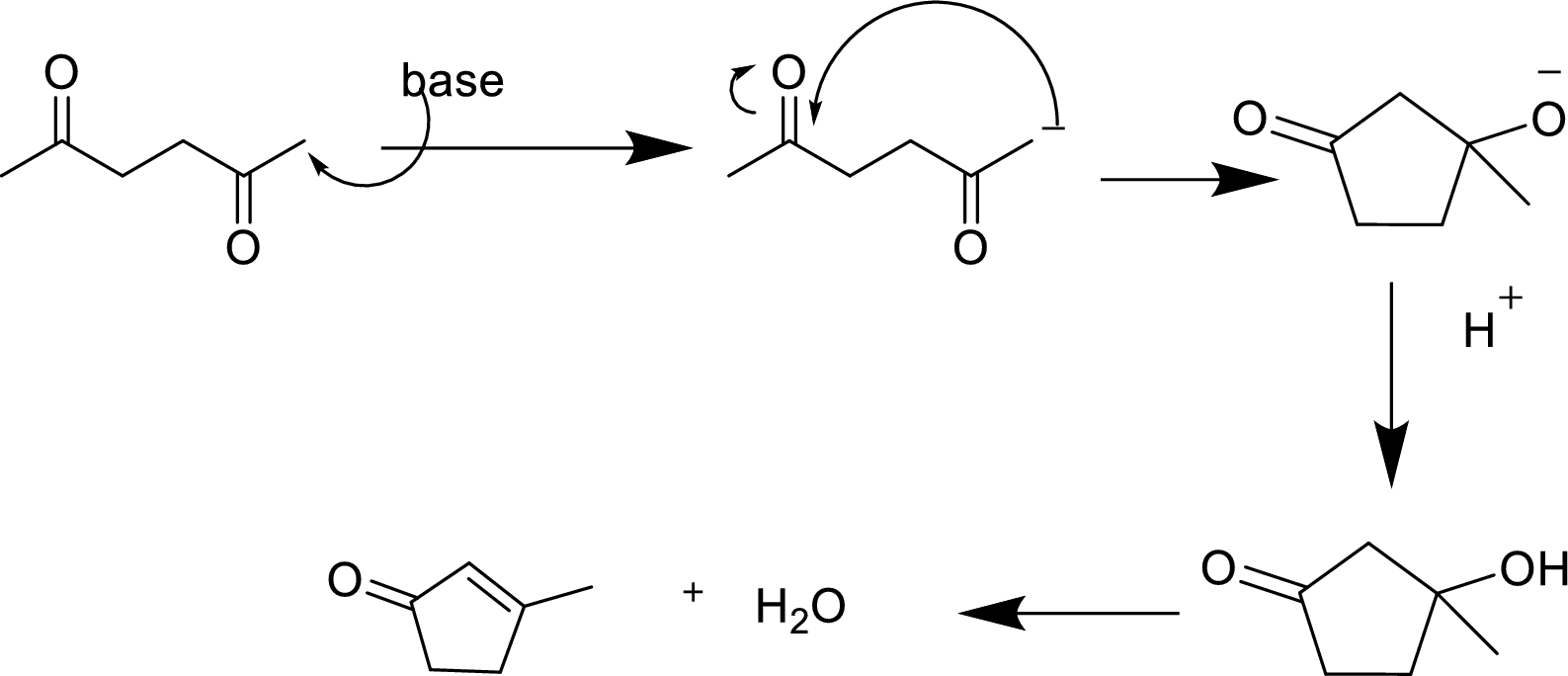
(c)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of aldehydes and ketones. Aldol reaction is a reversible reaction and occurs in the presence of a strong base like sodium hydroxide. One molecule (aldehyde or ketone) acts a nucleophile and attacks the electrophilic carbon center of the other molecule to give the addition product. The product is named
(c)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- What is the product formed when each dicarbonyl compound undergoes an intramolecular aldol reaction, followed by dehydration.arrow_forward2 H3C H3C H C→XT OH H3C The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a ß-hydroxy carbonyl compound. base :0: OH H H Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instruct ns H3C heat OH H3C :0: H + H₂O Harrow_forwardDraw the product formed when each dicarbonyl compound undergoes an intramolecular aldol reaction followed by dehydration, when possible.arrow_forward
- Draw the products (including the stereochemistry) formed in the following reaction.arrow_forwardDraw the product formed when each dicarbonyl compound undergoes anintramolecular aldol reaction followed by dehydration, when possible.arrow_forwardThe synthesis of carbohydrates can be particularly difficult because of the large number of chiral centers and OH functional groups present. Epoxides can be useful synthetic intermediates in carbohydrate syntheses. Draw the product of the following reaction of a Gilman reagent with each epoxidearrow_forward
- Give the products from the following aldol reactionarrow_forwardWhat is the product resulted due to the reaction of 2-hydroxy-3-methoxybenzaldehyde with ethyl bromoacetate in a basic solution indicating all the reagents and intermediates occurred during the reaction? What is the product obtained when product A reacted with ethyl acetate in the same basic solution?arrow_forwardDraw the aldol product formed from each compound.arrow_forward
- Give the reagents needed to complete each transformationarrow_forwardDraw the products obtained by reacting a sulfonitric mixture with: 4-nitrophenol, 2,4-dinitrophenol, phenol, 4-methoxyphenol, 4-chlorophenol.arrow_forward4) The following enone, 3-methylcyclopent-2-enone, was prepared through an intramolecular aldol condensation followed by a dehydration. Draw the starting diketone and the intermediate cyclic ß-hydroxy ketone. 3-methylcyclopent-2-enonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





