
Concept explainers
(a)
Interpretation: The class of given cycloocta-1,4-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(a)
Answer to Problem 15.24SP
The given compound is classified as isolated diene.
Explanation of Solution
The structure of given diene is shown in figure 1.
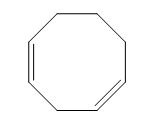
Figure 1
It is conferred from the above structure of
(b)
Interpretation: The class of given cycloocta-1,3-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(b)
Answer to Problem 15.24SP
The given compound is classified as conjugated diene.
Explanation of Solution
The structure of given diene is shown in figure 2.
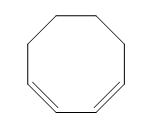
Figure 2
It is conferred from the above structure of
(c)
Interpretation: The class of given cycloocta-1,2-diene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(c)
Answer to Problem 15.24SP
The given compound is classified as cumulated diene.
Explanation of Solution
The structure of given diene is shown in figure 3.
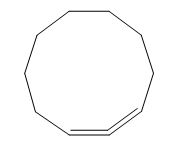
Figure 3
It is conferred from the above structure of
(d)
Interpretation: The class of given cycloocta-1,2,5,7-tetraene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(d)
Answer to Problem 15.24SP
The given compound is classified as conjugated polyene.
Explanation of Solution
The structure of given polyene is shown in figure 4.
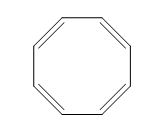
Figure 4
It is conferred from the above structure of
(e)
Interpretation: The class of given cyclohexa-1,3,5-triene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(e)
Answer to Problem 15.24SP
The given compound is classified as conjugated polyene.
Explanation of Solution
The structure of the given polyene is shown in figure 5.
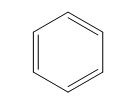
Figure 5
It is conferred from the above structure of
(f)
Interpretation: The class of given penta-1,2-4-triene as isolated, conjugated, cumulated, or a combination is to be stated.
Concept introduction: Conjugated systems have alternate single and double bonds that results in lower the energy and increase the stability of a molecule. Isolated dienes or polyenes are the compounds that comprise double bonds at the large distances separated by more than one single bond, whereas cumulated dienes or polyenes are the compounds that do not possess any single bond in between the two double bonds.
(f)
Answer to Problem 15.24SP
The given compound is classified as cumulated and conjugated polyene.
Explanation of Solution
The structure of given polyene is shown in figure 6.
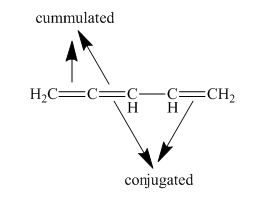
Figure 6
It is conferred from the above structure of
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry (9th Edition)
- 3. Draw the structure of the following alkenes. Some of these compounds can show isomerism, and some cannot. Indicate which among these can show cis and trans isomers. a. hex-3-ene b. buta-1,3-diene c. 2,3-dimethylpent-2-ene 4. Draw the structure of the following alkenes. Some of these compounds can show isomerism, and some cannot. Indicate which among these can show cis and trans isomers. a. 3-ethylhexa-2,4-diene b. pent-1,3-diene c. 3,7-dichloroocta-2,5-dienearrow_forwardC. cyclohexyl alcohol D. isoamyl alcohol 38. An aromatic ring should satisfy Huckel's rule, wherein the number of electrons participating in the cyclic conjugation should be equal to 4n +2. Which of the following cyclic structures does NOT obey Huckel's rule? A. Cyclobutadienyl dianion B. Tetrahydrofuran ring C. Pyrimidine ring structure D. Cyclopropene structure 39. Why is ethanal incapable of forming a hydrogen bond with another ethanal molecule despite exhibiting a bond dipole in the C=O bond? A. The oxygen atom is partially reactive due to the strong bond dipole. B. The carbon atom does not form a highly polar bond with hydrogen. C. The oxygen atom is sp2 hybridized with almost 67% p character. D. Oxygen exhibits resonance with carbon leading to bond stability. 40. What is the role of H+ ions in the nucleophilic substitution of alcohols using hydrogen halides? A. Protonation of -OH group B. Stabilization of carbocation C. Activation of oxygen radical D. Removal of an alkyl group…arrow_forwardWrite the equation for the following reactions: a. 2-butene + HCI b. 1,2-dichloro-1-propene + water c. Propyne + oxygen d. 1, 3-pentadiene + bromine e. cis-3,3,4-trimethyl-1-hexene + H₂ parrow_forward
- O A. 1,1-dimethyl-1,4-pentadiene B. 2-methyl-2,5-hexadiene OC.5,5-dimethyl-1,4-pentadiene O D.5-methyl-1,4-hexadienearrow_forwardDraw the skeletal structures for the compounds in a. (Z)-1,3,5-tribromo-2-pentene b. (Z)-3-methyl-2-heptenec. (E)-1,2-dibromo-3-isopropyl-2-hexene d. vinyl bromidee. 1,2-dimethylcyclopentene f. diallylaminearrow_forwardDraw the structures for penta-1,4-diene and cyclopenta-1,3-diene.arrow_forward
- Draw structures corresponding to the following IUPAC names: a. 2-methylhexa-1,5-diene b. 3,4-diisopropy;-2,5-dimethyl-3-hexene c. 3-ethyl-2,2-dimethylhept-3-ene d. (4E)-2,4-dimethyl-1,4-hexadiene e. 2,3,3-trimethylocta-1,4,6-triene f. (3E,5Z)-2,6-dimethyl-1,3,5,7- octatetraenearrow_forwardReagents a. C6H5CHO b. NaOH, ethanol h. BrCH2CH=CH2 i. Na* OEt, ethanol j. Br2, H* k. K* t-BuO c. Pyrrolidine, cat. H* d. H2C=CHCN e. H3O* f. I. CH2(CO2ET)2 -CH2CH2CN LDA m. heat g. ELOC(=0)CO2ET Select reagents from the table to synthesize this compound from cyclopentanone. Enter the letters of the chosen reagents, in the order that you wish to use them, without spaces or punctuation (i.e. geda).arrow_forwardDescribe (meaning what you would do and observe) simple (not including melting points, boiling points, cleaving reactions, or instrumental analyses) chemical tests(if any) that would distinguish between the compounds named below. [hint doing a table may be helpful] A. 1,3-pentadiene and 1-pentyne B. Ally bromide and 2,3- dimethyl-1,3-butadienearrow_forward
- Which of the following molecules is antiaromatic? A. cycloheptatrieneB. Cyclopentadienyl cationC. cyclopentadienyl anionD. cycloheptatrienyl cationE. silopentadienearrow_forward9. The catalyst used for vinyl chloride production from ethylene is: a. FeCl, b. Ethylene dibromide c. HPO. d. Both (a) or (b) 10. LPG is mainly used for production of a- Ethylene. c-Butadiene. b- Propylene. d- All of these.arrow_forwardShow how to convert 1- Butene into these compounds. a. Butane b. 2- Butanol c. 2- Bromobutane d. 1,2- Dibromobutanearrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

