
Concept explainers
Interpretation:
According to the terpene rule, the structure of caryophyllene needs to be explained.
Concept introduction:
Terpene rule or isoprene rule states that terpenoids structures are obtained by linking isoprene unit. Isoprene (2-methyl-1, 3-butadiene) is the monomer of natural rubber.
Answer to Problem 3Q
The structure of caryophyllene fits in the terpene rule as caryophyllene contains a moiety which is similar to the polyisoprene unit.
Explanation of Solution
The structure of caryophyllene is represented as follows:

The
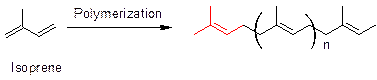
Polymerization of isoprene leads to a basic structure that is present in all terpenoids (shown in red). Similarly, caryophyllene also has a similar moiety (shown red) as present in terpenoids. Thus, the structure of caryophyllene fits in the terpene rule.
Want to see more full solutions like this?
Chapter 13 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Imagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) In analyzing the products, you discover that the substitution pattern resulting from the reaction with phenol differs from the reaction with nitrobenzene. Explain this difference in substitution patterns using one or more judiciously selected reaction mechanisms or portions of mechanisms.arrow_forwardAssuming you are a chemist and you need to extract ethanolic acid in a benzene solution. Propose and briefly discuss how to extract the ethanolic acid in the benzene solution. Include the reactions involved in each step, if any.arrow_forwardOrganic chemistry:Discuss the Grignard synthesis of triphenyl methanol and benzoic acid .arrow_forward
- 1. Show the mechanism using curly arrows for the transformation of benzoic acid to benzoyl chloride using SOCI, as a reagent as shown below. Benzoic acid Benzoyl chloride 2. Carboxylic acid and their derivatives readily react with alcohols. (i) Name the reaction and give the generic name of the product of such a reaction. (ii) The reaction of salicylic acid with acetic anhydride is an example of such a reaction as in (i) above. Show the mechanism of the reaction. HO H* HO salicylic acid acetic anhydride acetyl salicylic acid (aspirin)arrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardSuppose you took your two compounds, dissolved them in tertbutyl methyl ether and then added them to a separatory funnel. Now suppose you add in aqueous sodium bicarbonate and vigorusly shake the contents of the separatory funnel. ethanol will be dissolved in tert butyl methyl ether ether and valeric acid will be dissolved in the aqueous sodium bicarbonate layer. Draw the EXACT chemical structure that will exist in each of the layers after shaking with sodium bicarbonate. (Is it neutral or charged?)arrow_forward
- In this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardDraw structural formulas for the a,ß-unsaturated aldehyde or ketone and the lithium diorganocuprate (Gilman reagent) that could be used in a synthesis of the compound shown below. You do not have to consider stereochemistry. In cases where there is more than one answer, just give one. If a structure has a copper-lithium bond, draw the lithium ion in a separate sketcher. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple reactants using the + sign from the drop-down menu. • 0 Call ? [Farrow_forward
- Give an implication or an inference to the following reaction below 1. effervescence where formed when aniline was added to HCl 2. there are bubbles formed when aniline is added to nitrous acid 3. the orange precipitate when aniline was subjected into Reaction with azo dye test (diazonium solution + β- naphthol + NaOH + distilled water)arrow_forwardDraw structural formulas for the a,ß-unsaturated aldehyde or ketone and the lithium diorganocuprate (Gilman reagent) that could be used in a synthesis of the compound shown below. H3C. You do not have to consider stereochemistry. • In cases where there is more than one answer, just give one. If a structure has a copper-lithium bond, draw the lithium ion in a separate sketcher. •Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. •Separate multiple reactants using the + sign from the drop-down menu. ? [P ChemDoodle Previous Next>arrow_forwardWhat is the theoretical yield and limiting reagent for the reaction of benzophenone with sodium borohydride to form diphenyl methanol as described in the experiment provided if you begin with 1.75g of benzophenone in 25 mL of 95% ethanol and add 0.75 g of sodium borohydride portion wise over 45 minutes?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





