Intensity (PA) Intensity (PA) 400000 350000 300000 250000 200000 150000 100000 50000 0 -50000 700000 600000 500000 400000 300000 200000 100000 0 -100000 0 0 0.5 0.5 1 Reaction of 1-hexene with HBr 1 1 2 1.5 2 Reaction of 1-hexene with HBr Peak Number Retention Time (min) Peak Area %) 2.231 48.611 3.428 51.389 1 2 3 1.5 2.5 Time (min) Reaction of 3,3-dimethyl-1-butene with HBr 2 2.5 3 Time (min) Reaction of 3,3-dimethyl-1-butene with HBr Peak Number Retention Time (min) Peak Area %) 2.214 2.967 3.876 3 26.996 23.451 49.553 3.5 3.5 4 4 4.5 4.5
Intensity (PA) Intensity (PA) 400000 350000 300000 250000 200000 150000 100000 50000 0 -50000 700000 600000 500000 400000 300000 200000 100000 0 -100000 0 0 0.5 0.5 1 Reaction of 1-hexene with HBr 1 1 2 1.5 2 Reaction of 1-hexene with HBr Peak Number Retention Time (min) Peak Area %) 2.231 48.611 3.428 51.389 1 2 3 1.5 2.5 Time (min) Reaction of 3,3-dimethyl-1-butene with HBr 2 2.5 3 Time (min) Reaction of 3,3-dimethyl-1-butene with HBr Peak Number Retention Time (min) Peak Area %) 2.214 2.967 3.876 3 26.996 23.451 49.553 3.5 3.5 4 4 4.5 4.5
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
CANNOT BE HAND-WRITTEN!
SEE IMAGE*
3C. Below are the gas chromatograph results for the reaction of 1-hexene with HBr and the reaction of 3,3-dimethyl-1-butene with HBr performed in the experiment. The peak for the solvent, diethyl ether, has been cut out of the chromatograph so only the product peaks are displayed.
Identify the peaks in both gas chromatographs
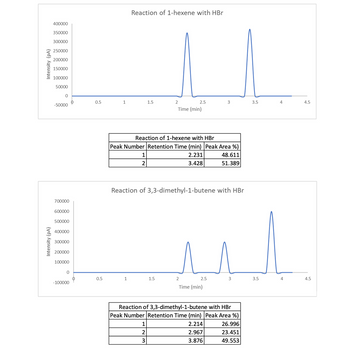
Transcribed Image Text:Intensity (PA)
Intensity (PA)
400000
350000
300000
250000
200000
150000
100000
50000
0
-50000
700000
600000
500000
400000
300000
200000
100000
0
-100000
0
0
0.5
0.5
1
Reaction of 1-hexene with HBr
1
1
2
1.5
2
Reaction of 1-hexene with HBr
Peak Number Retention Time (min) Peak Area %)
2.231
48.611
3.428
51.389
1
2
3
1.5
2.5
Time (min)
Reaction of 3,3-dimethyl-1-butene with HBr
2
2.5
3
Time (min)
Reaction of 3,3-dimethyl-1-butene with HBr
Peak Number Retention Time (min) Peak Area %)
2.214
2.967
3.876
3
26.996
23.451
49.553
3.5
3.5
4
4
4.5
4.5
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
