
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.69P
Devise a synthesis of each compound from
a. 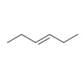 b.
b. 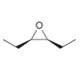 c.
c. 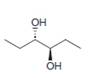
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Synthesize each compound from cyclohexanol using any other organic or inorganic compounds.
CH,OH
a.
g.
(Each cyclohexane ring must
come from cyclohexanol.)
COOH
b.
d.
h.
сно
CHs
(Each cyclohexane ring must
come from cyclohexanol.)
Design a synthesis of each compound from alcohols having four or fewer
carbons, acetylene, and ethylene oxide as the only organic starting
materials. You may use any other inorganic reagents you choose.
a.
b.
C.
CO₂H
xx
но
d.
OH
Devise a synthesis of each compound from benzene. You may use
alcohols with one or two carbons and any inorganic reagents.
a.
qe
Br
H
Br
b.
C.
N
OH
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which of the following solutions has the higher molarity? 10 ppm KI in water or 10,000 ppb KBr in water 0.25 ma...
CHEMISTRY-TEXT
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Design a synthesis of each compound from alcohols having four or fewer carbons, acetylene, and ethylene oxide as the only organic starting materials. You may use any other inorganic reagents you choose. a. b. C. CO₂Harrow_forward11. Devise a synthesis of each compound from benzene, any organic alcohols having four or fewer carbons, and any required reagents.arrow_forwardDevise a synthesis of each compound from the indicated starting material. You may use any other needed organic or inorganic reagents.arrow_forward
- Devise a synthesis of each compound from phenol (C6H5OH) and any other organic or inorganic reagents.arrow_forwardSynthesize each compound from cyclohexanol using any other organic or inorganic compounds. a. ہیں۔ بھی مله b. C. OH d.arrow_forwardDevise a synthesis of each compound from benzene. You may also use any organic compounds having four carbons or fewer, and any required inorganic reagents. Co,CH3 a. b.arrow_forward
- 3.provide the starting materials needed to synthesize each compound.arrow_forwardDevise a synthesis of each compound using CH3CH2CH=CH2 as the starting material. You may use any other organic compounds or inorganic reagents.arrow_forwardDevise a synthesis of each compound from the indicated starting material, organic compounds containing one or two carbons, and any other required reagents.arrow_forward
- Show how to bring about each conversion in good yield. a. b. C6H5 Cl OH COOH C6H5 COOHarrow_forwardCompound that is most easily hydrolyzed by acid in water `NH A. В. F C. D.arrow_forwardIdentify the major organic product(s) of the following reaction. x A. B. 1) KOH D. ه (2 مسلسل موسلی yasarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY