Indicate the number of signals and the multiplicity of each signal in the 1H NMR spectrum of each of the following compounds:

“Since you have posted a question with multiple sub-parts, we will solve the first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.”
1H NMR is basically an analytical method utilized in organic chemistry to study the unknown organic molecules' structure and composition. The chemical shift gets defined by utilizing existing protons' chemical environment. Distinct proton depicts distinct signals and their multiplicity gets determined by applying (n+1) rule.
In the compound (a), all the protons that are involved in furnishing signals along with the multiplicity are symbolized. The identical protons get symbolized using the same alphabet. The number of signals and their multiplicity in 1H-NMR is shown below.
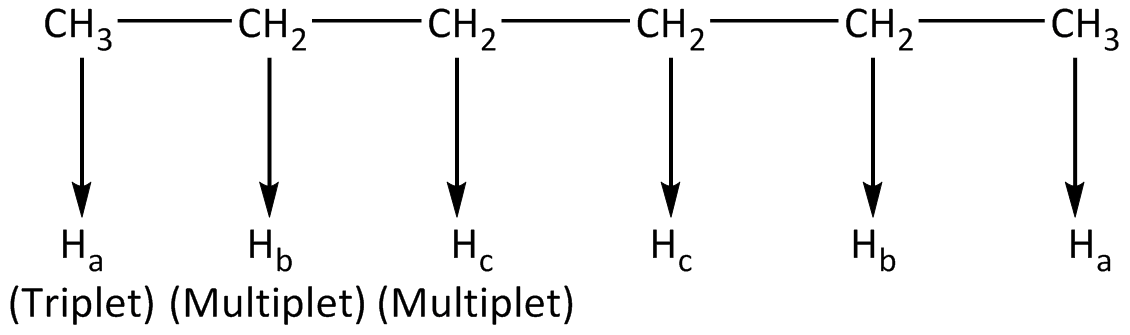
In the compound (a), a total of 6- types of protons are available in which identical protons will give 1-signal each. Thus the compound depicts total 3-signals in 1H NMR spectrum.
Step by step
Solved in 4 steps with 3 images









